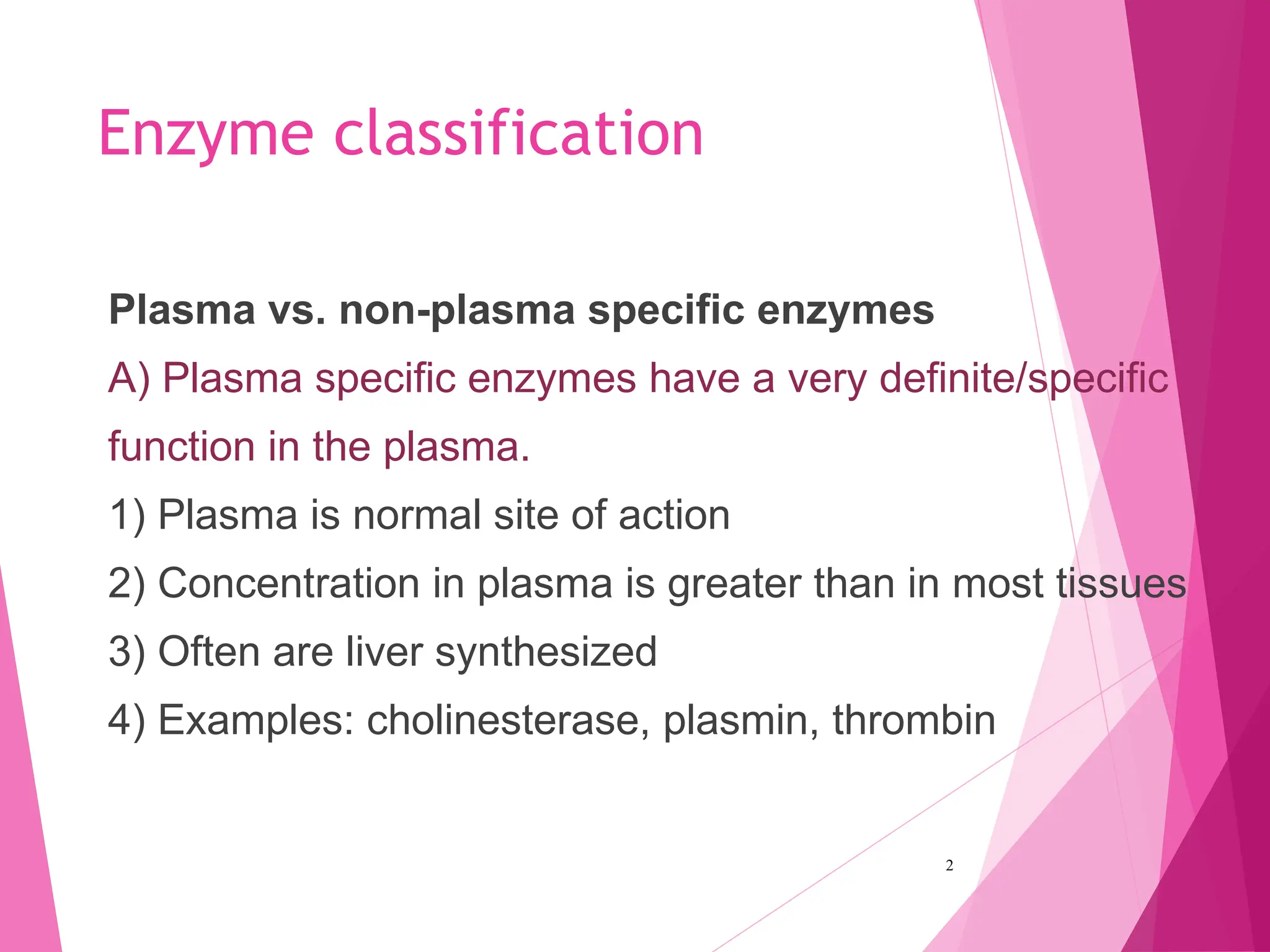
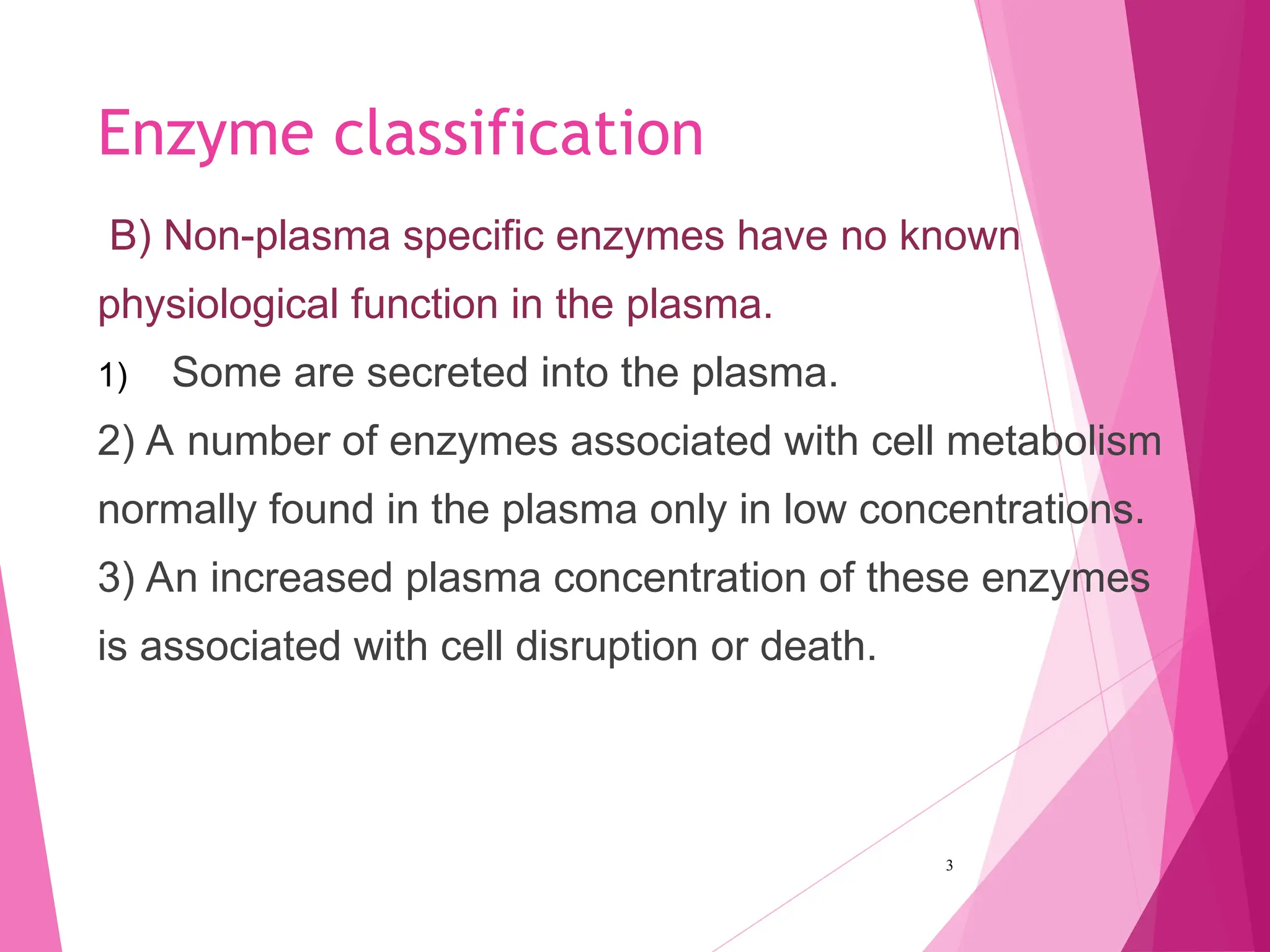
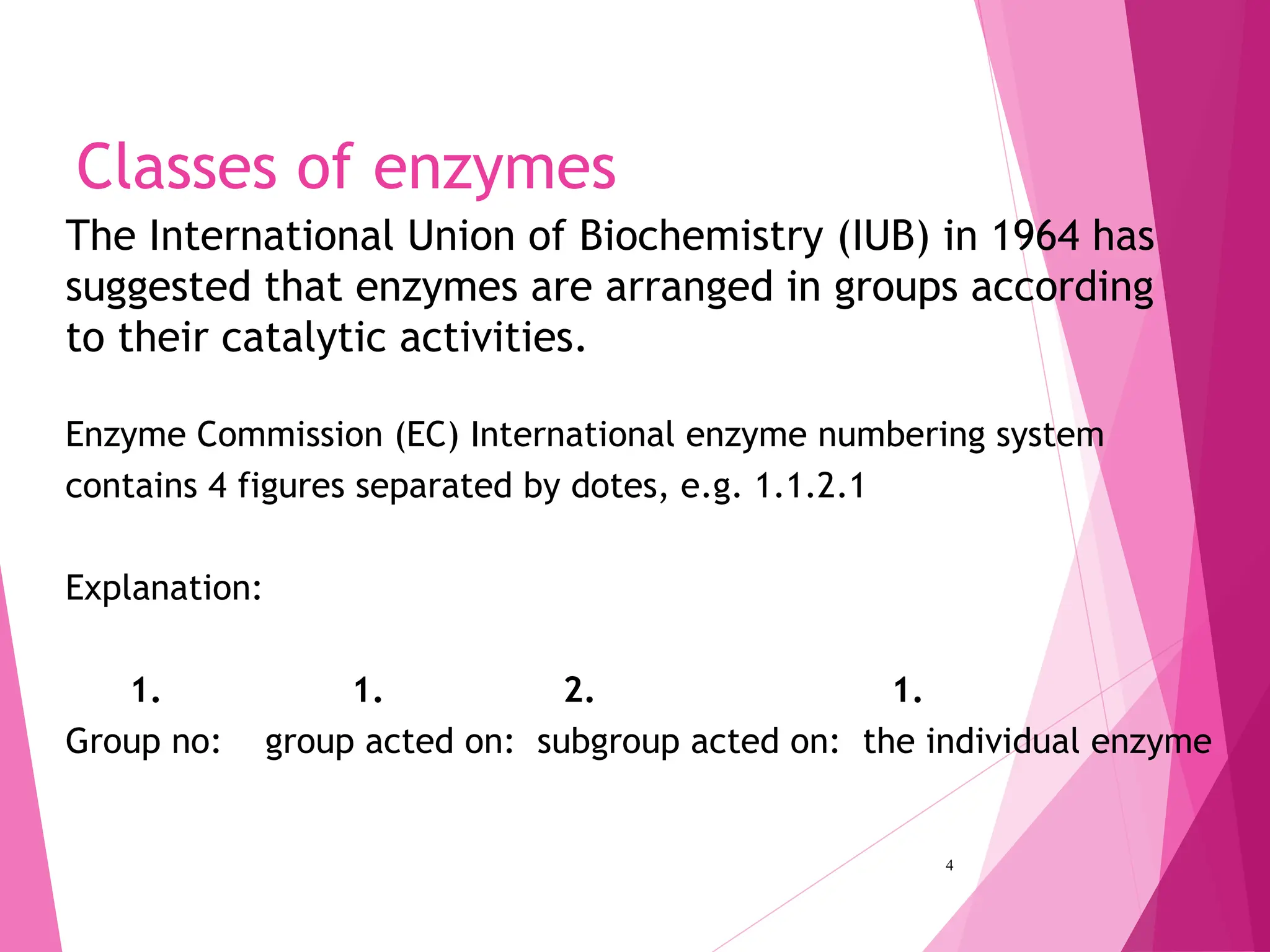
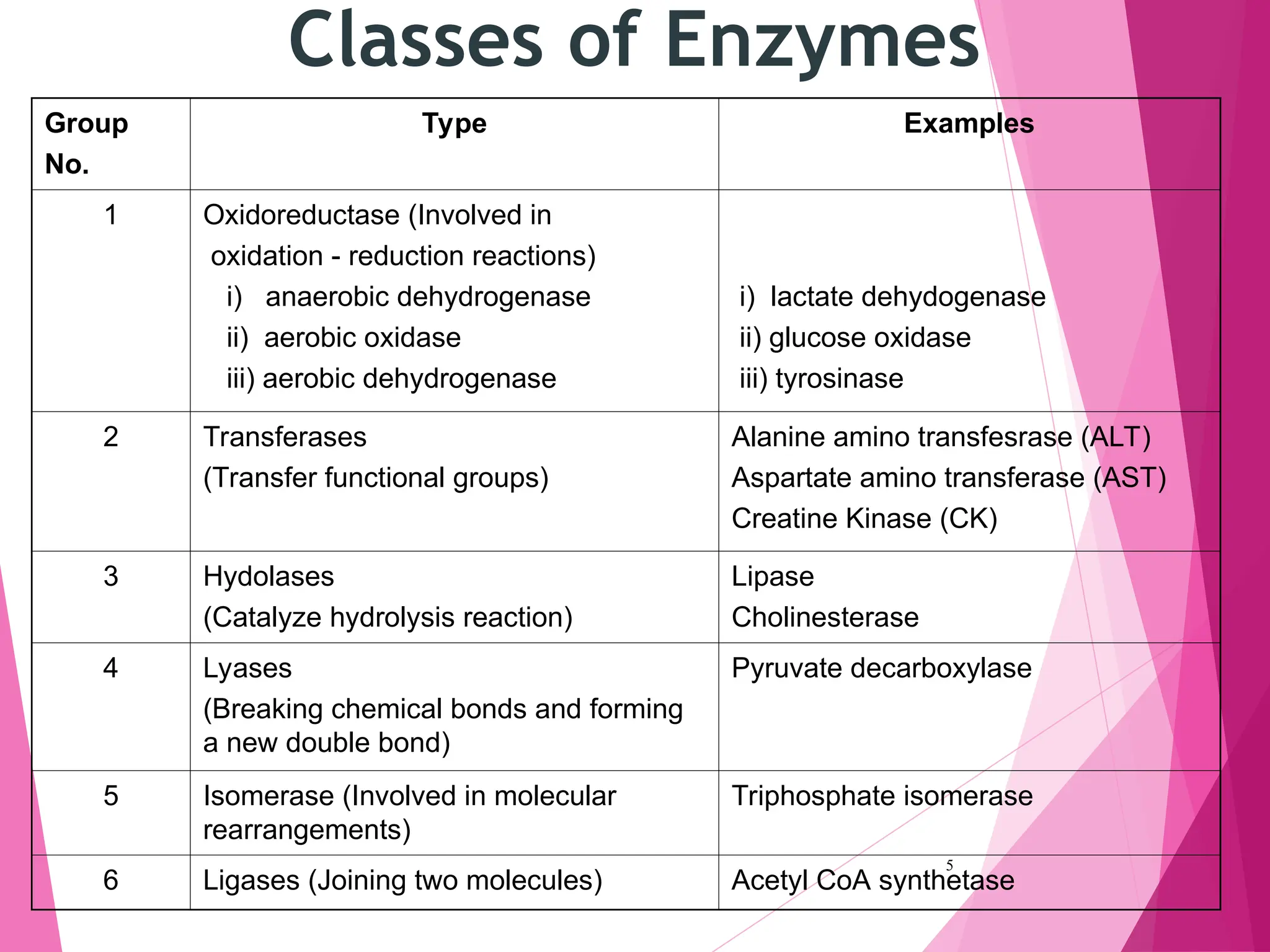
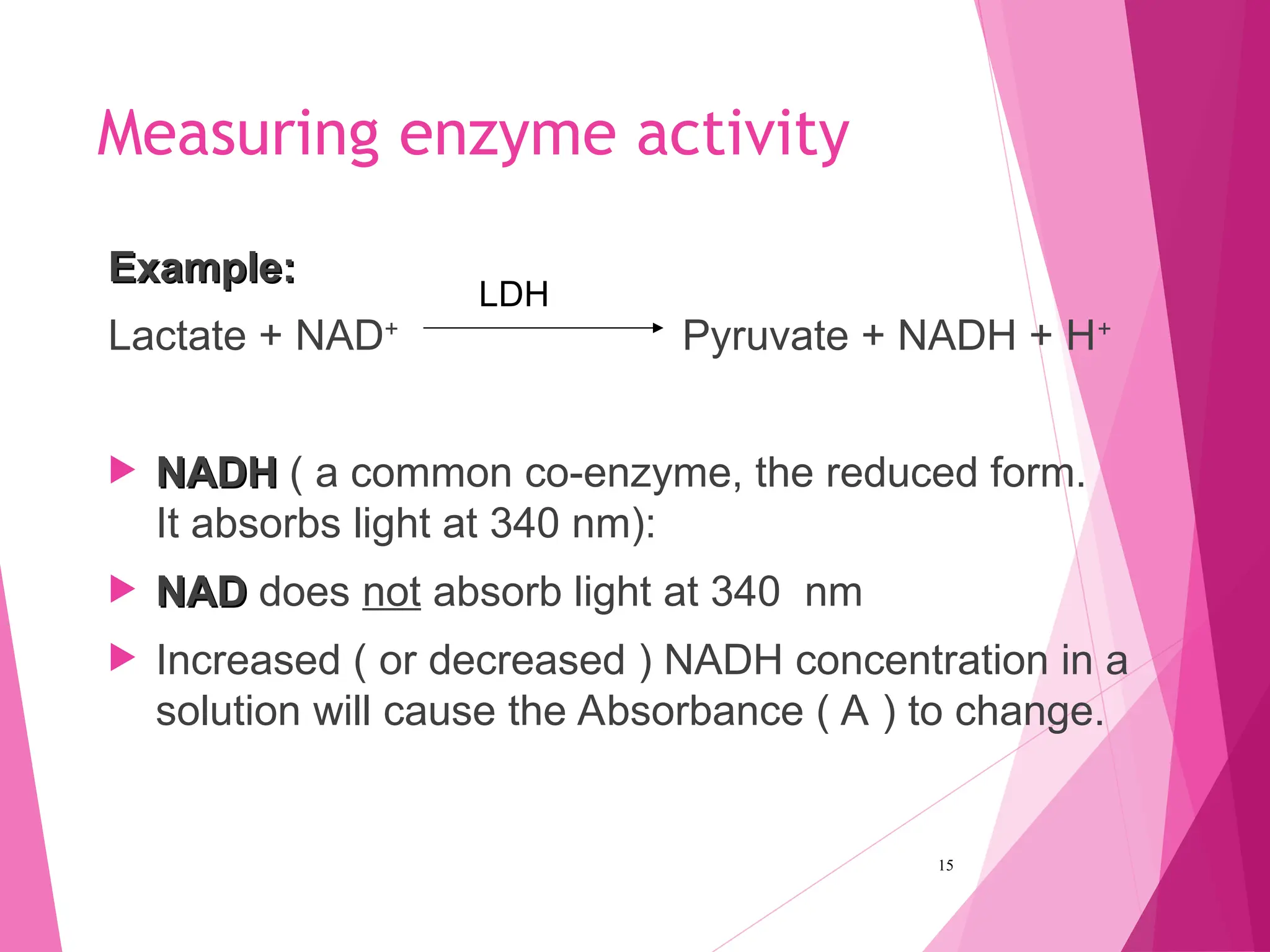
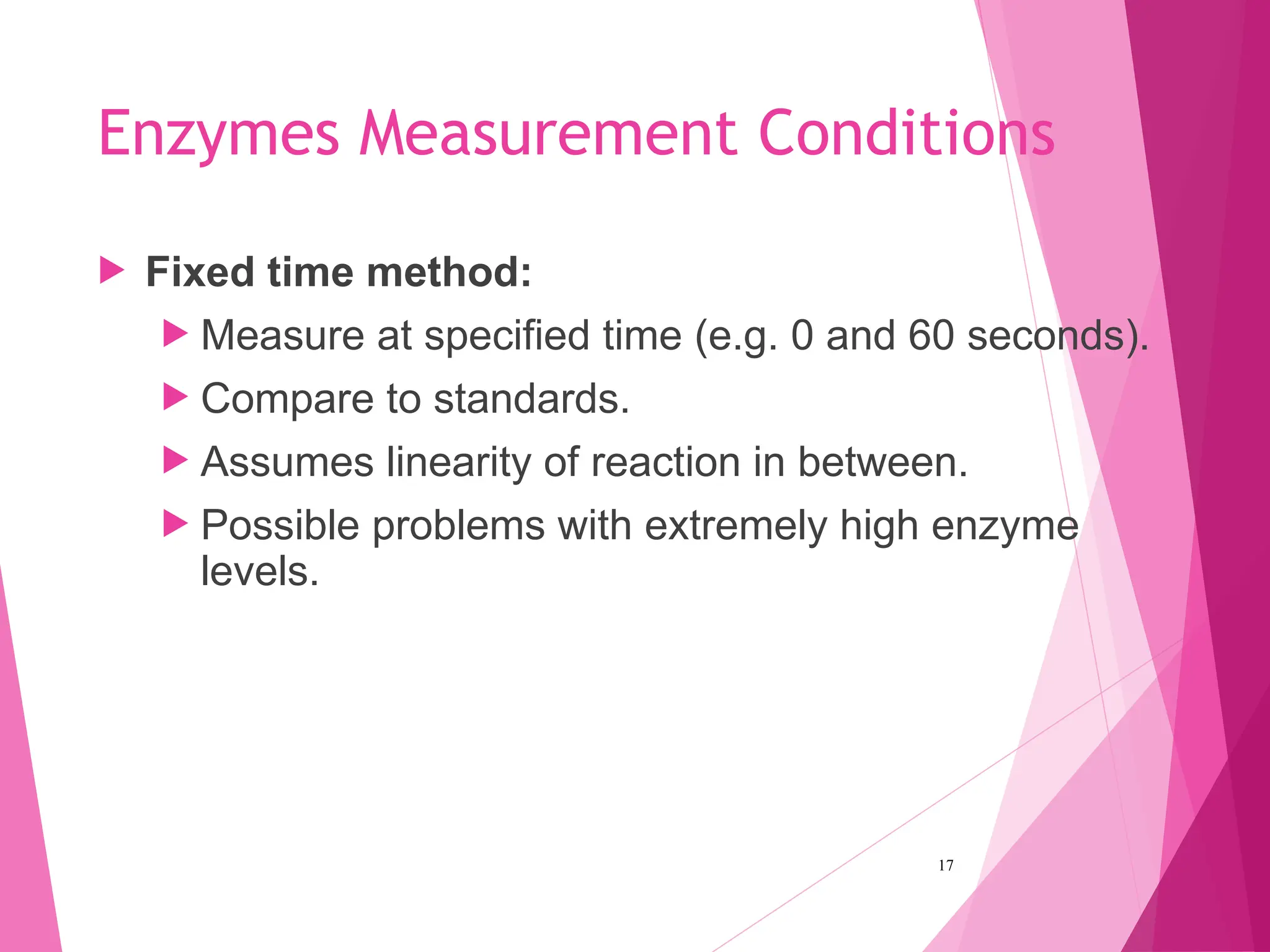
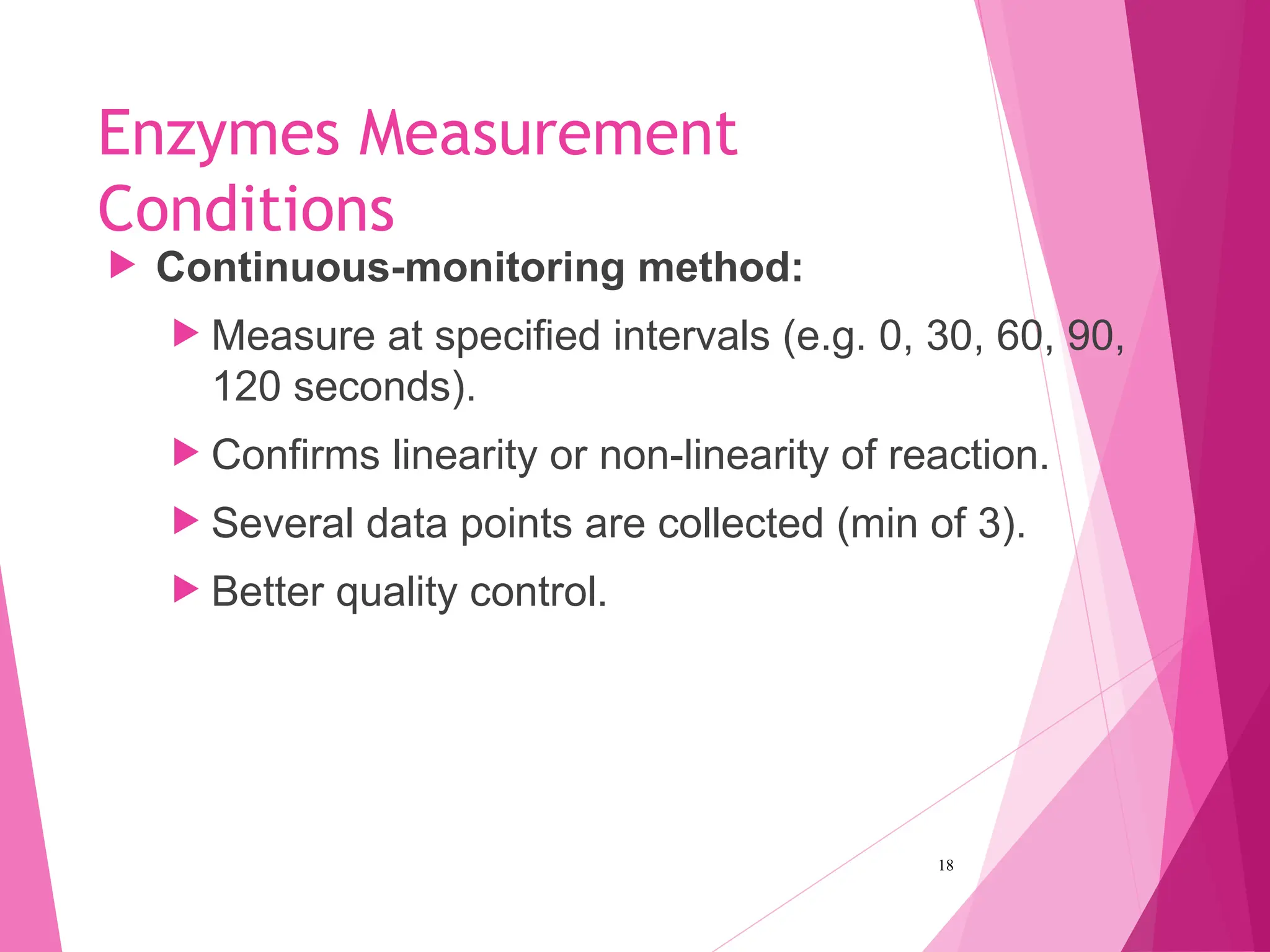
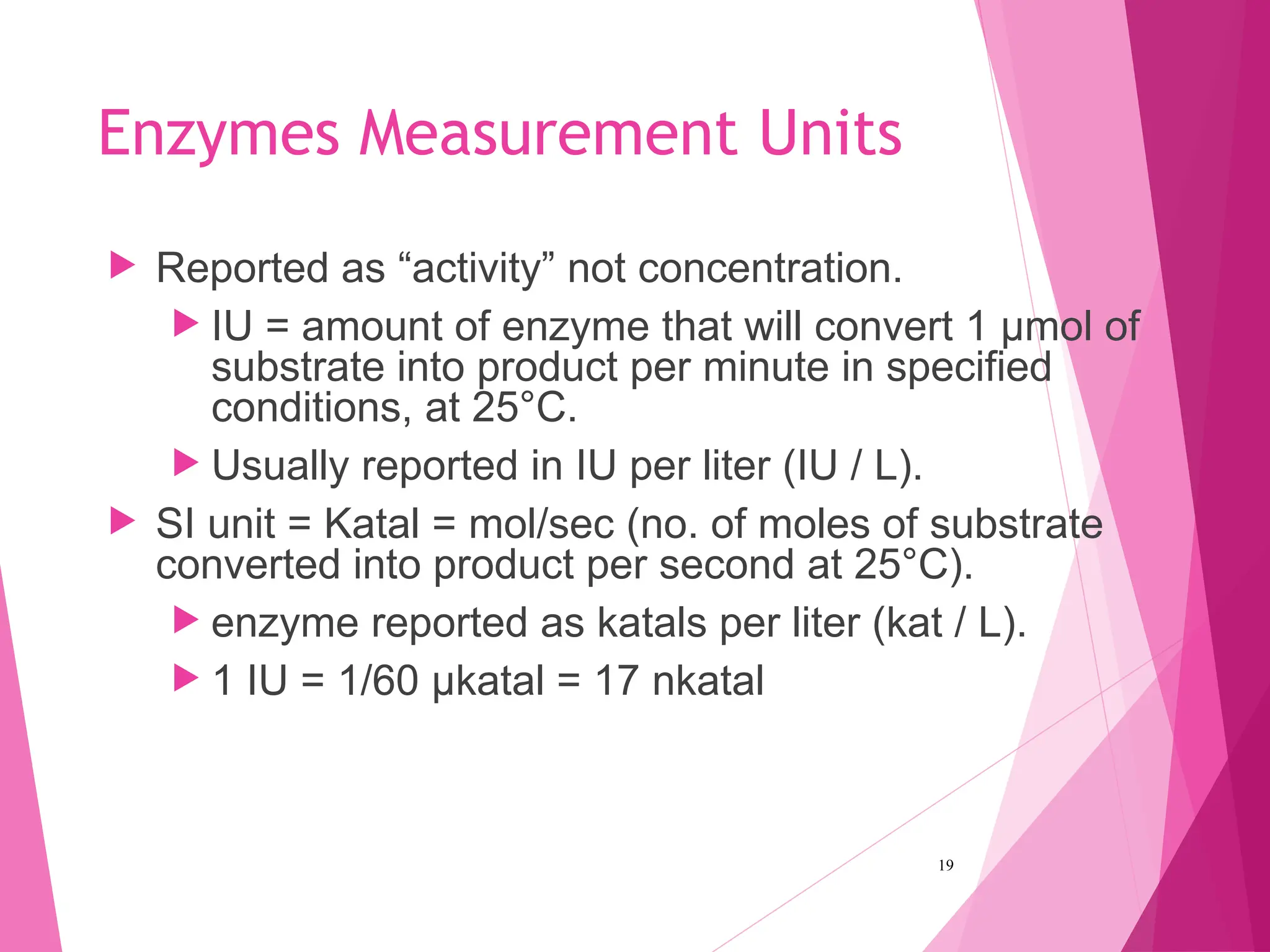
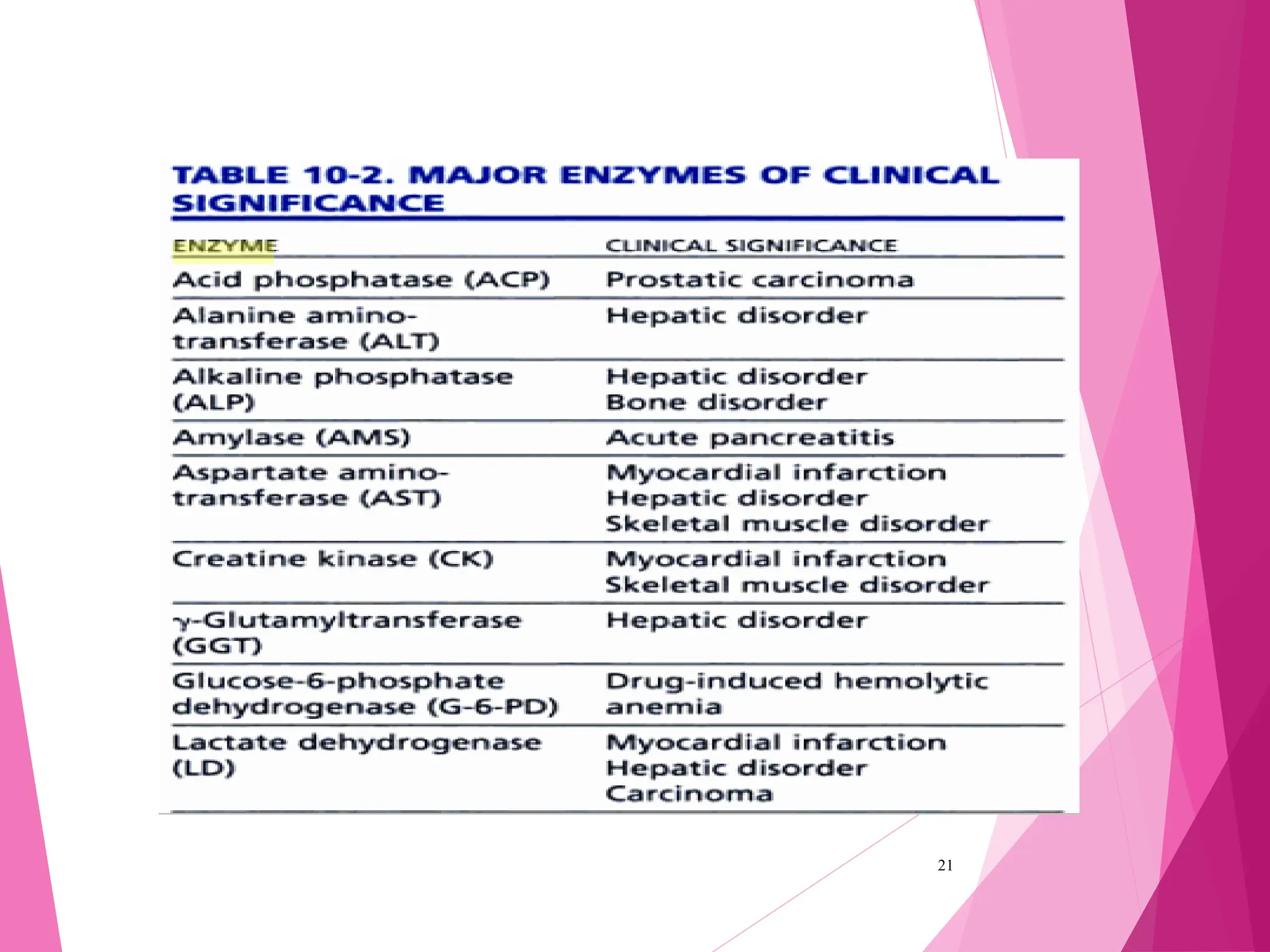
The document discusses the classification of enzymes into plasma-specific and non-plasma-specific categories, highlighting their functions and significance in clinical diagnostics. It details the methodologies for enzyme measurement in clinical laboratories, including precautions to ensure accuracy, as well as the interpretation of enzyme activities reflecting physiological conditions. Additionally, it outlines the parameters affecting enzyme assays and the importance of establishing lab-specific normal ranges for accurate diagnostic assessments.