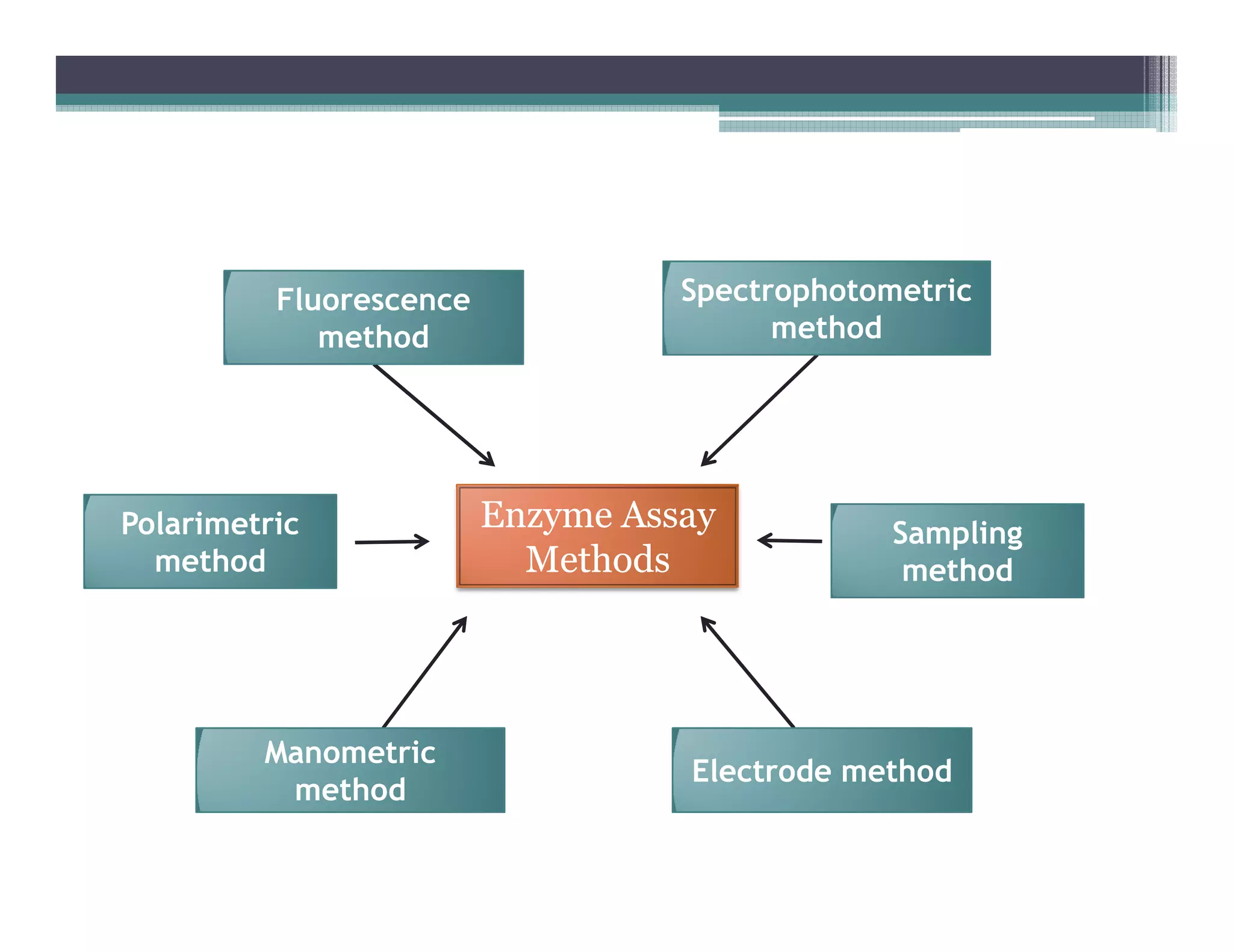
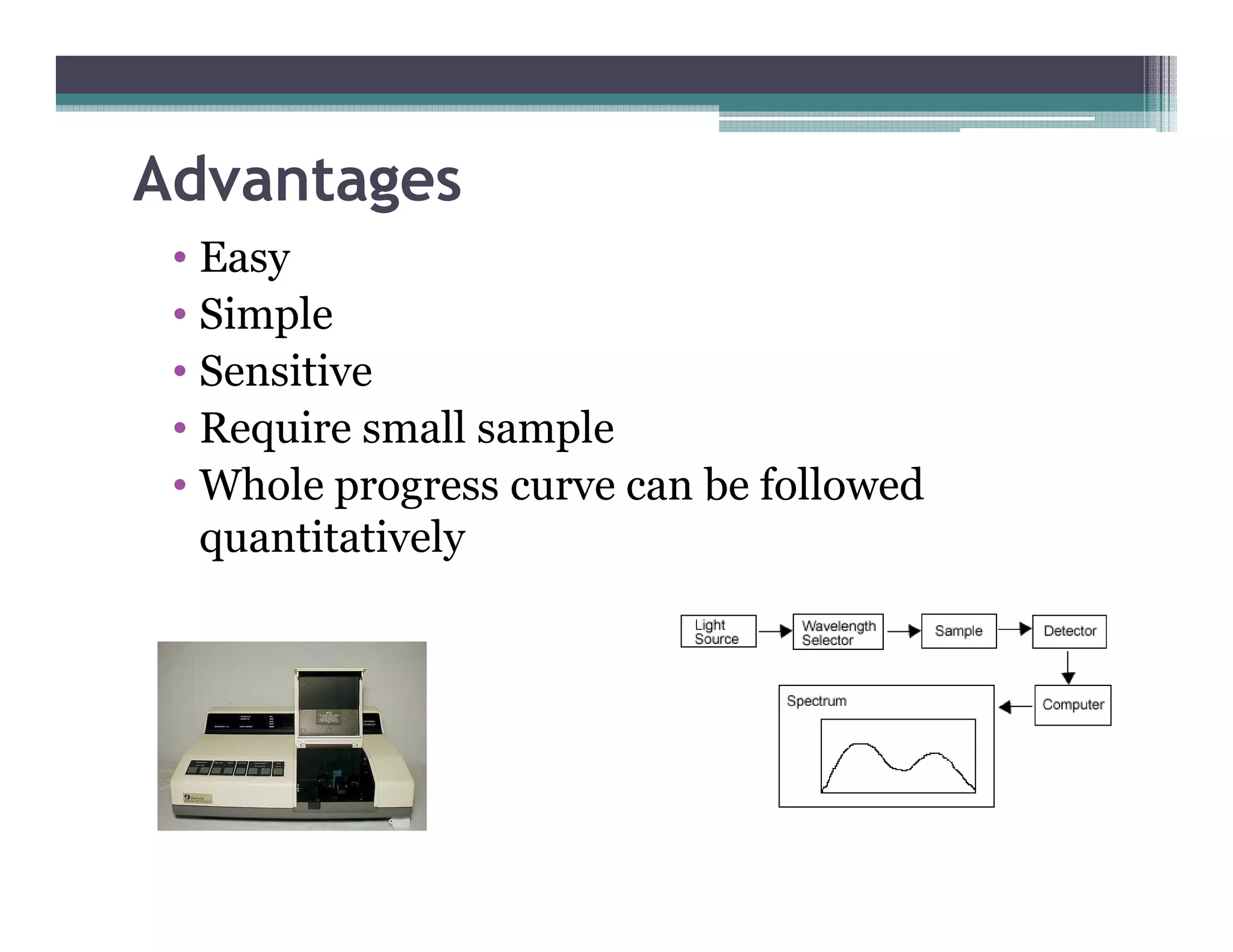
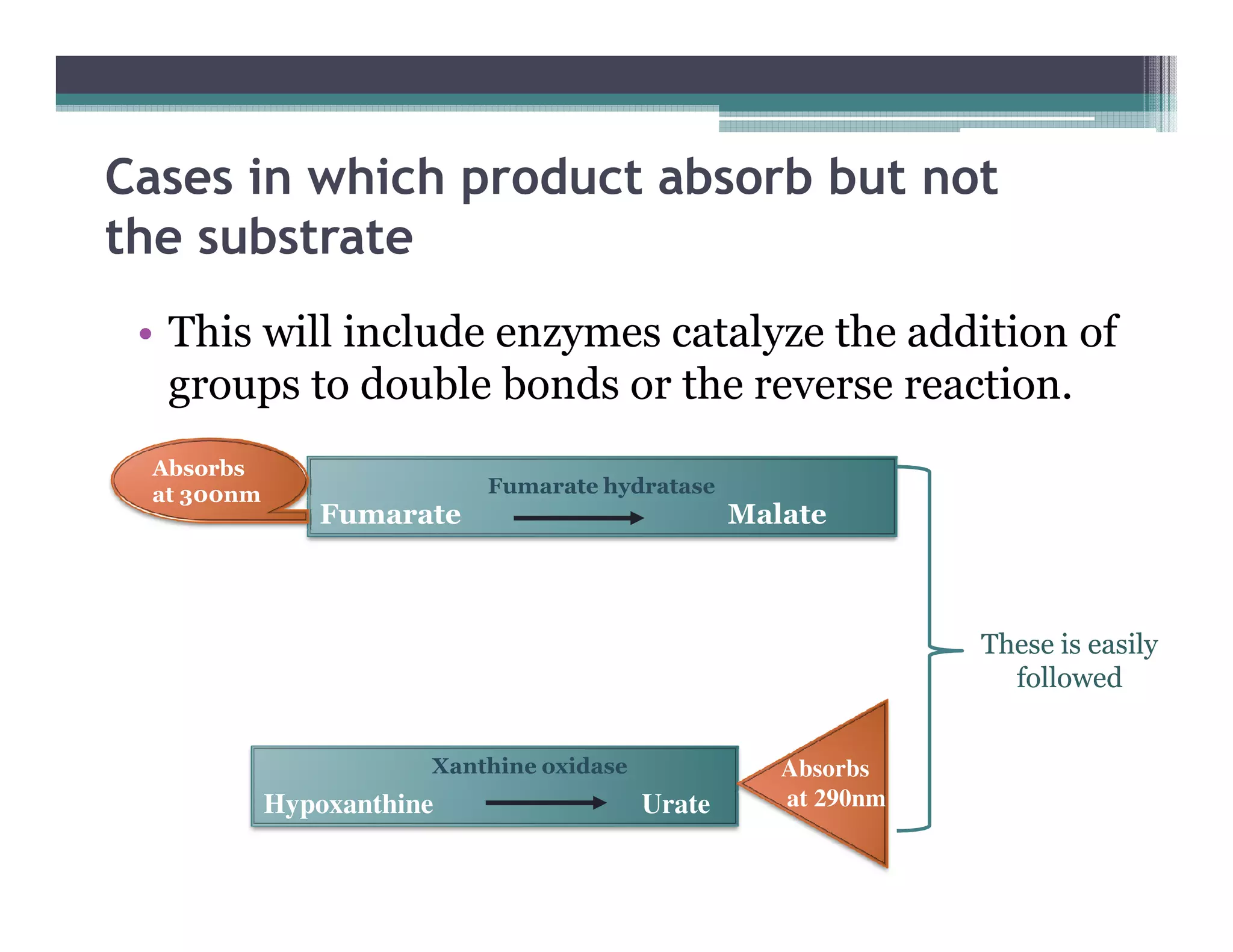
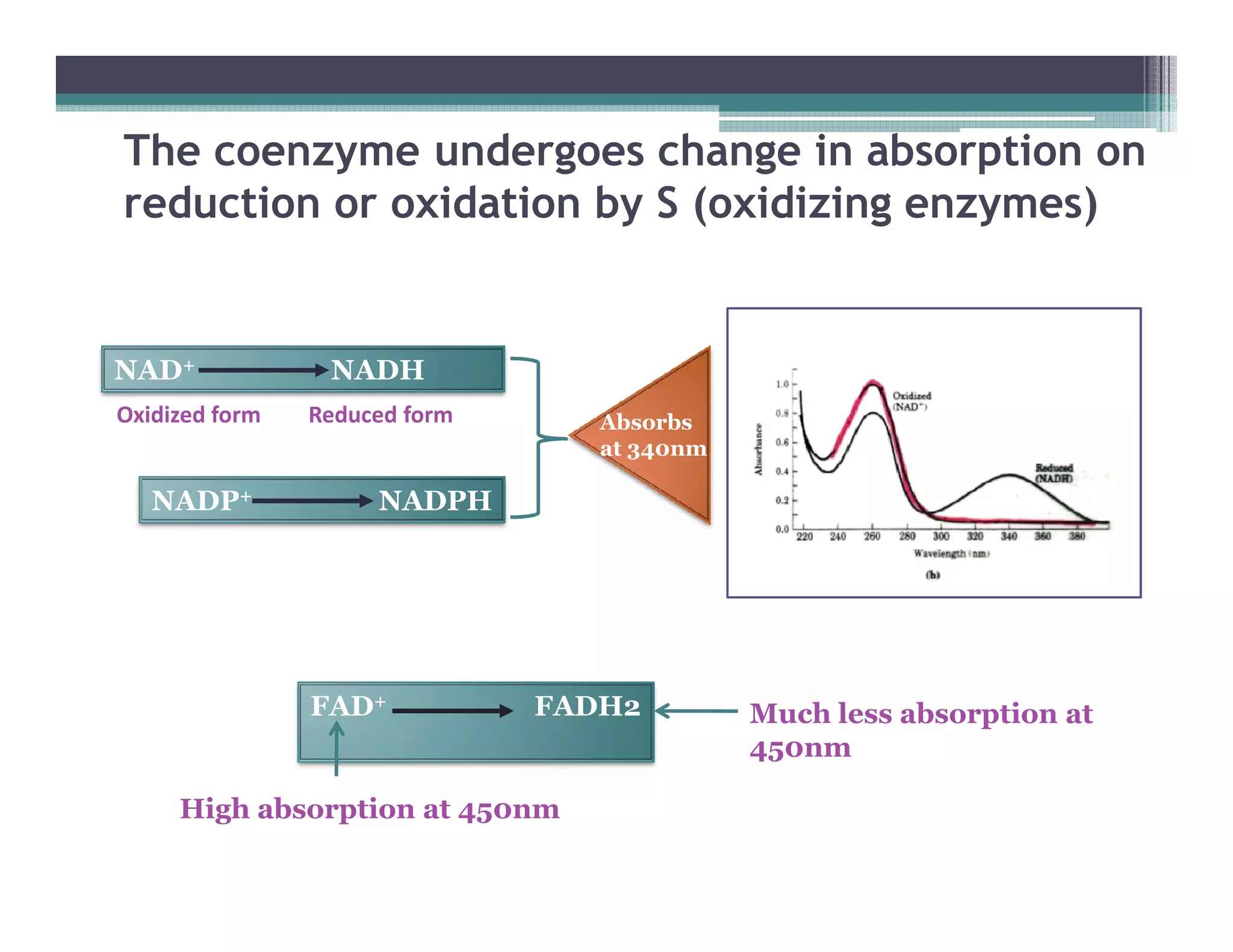
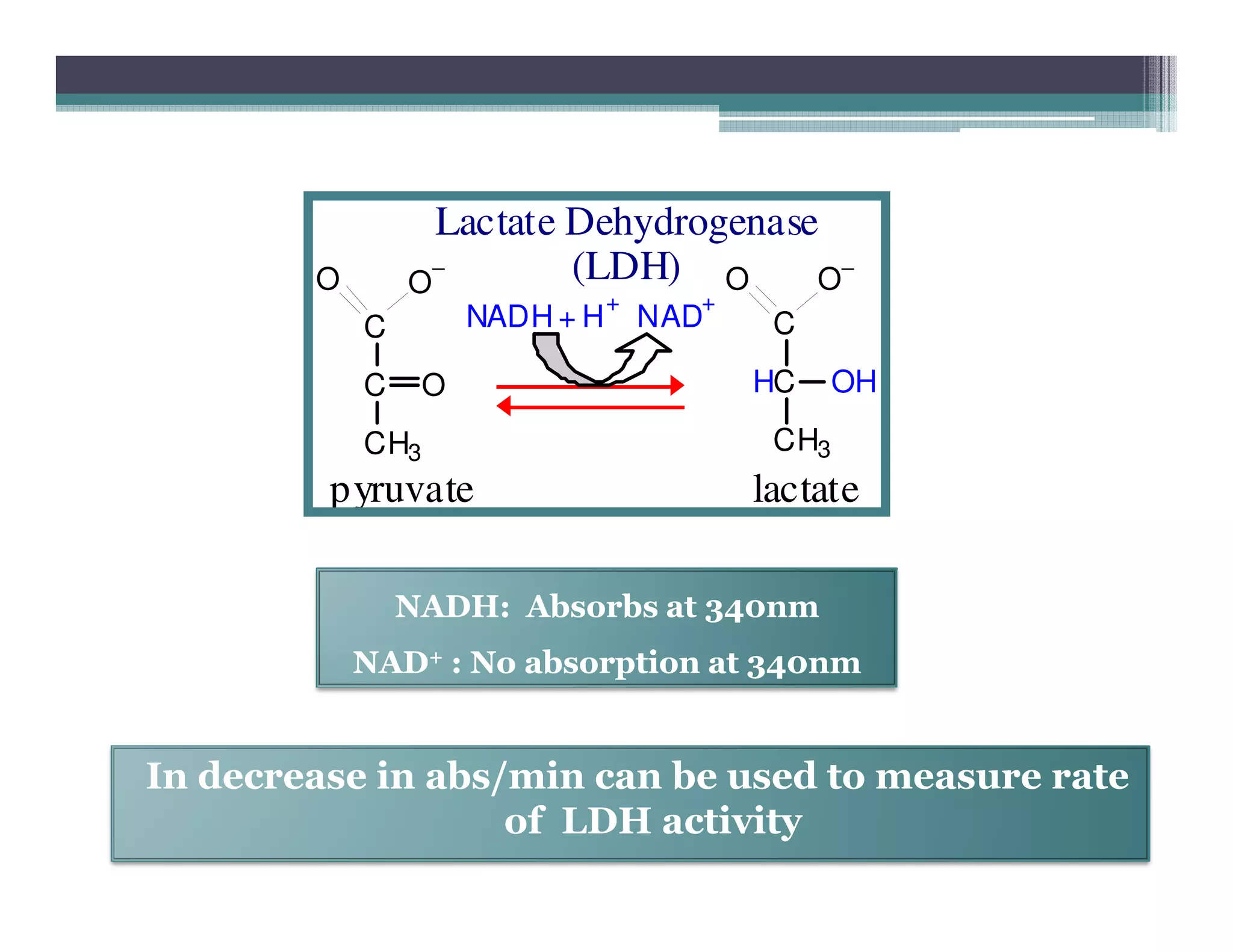
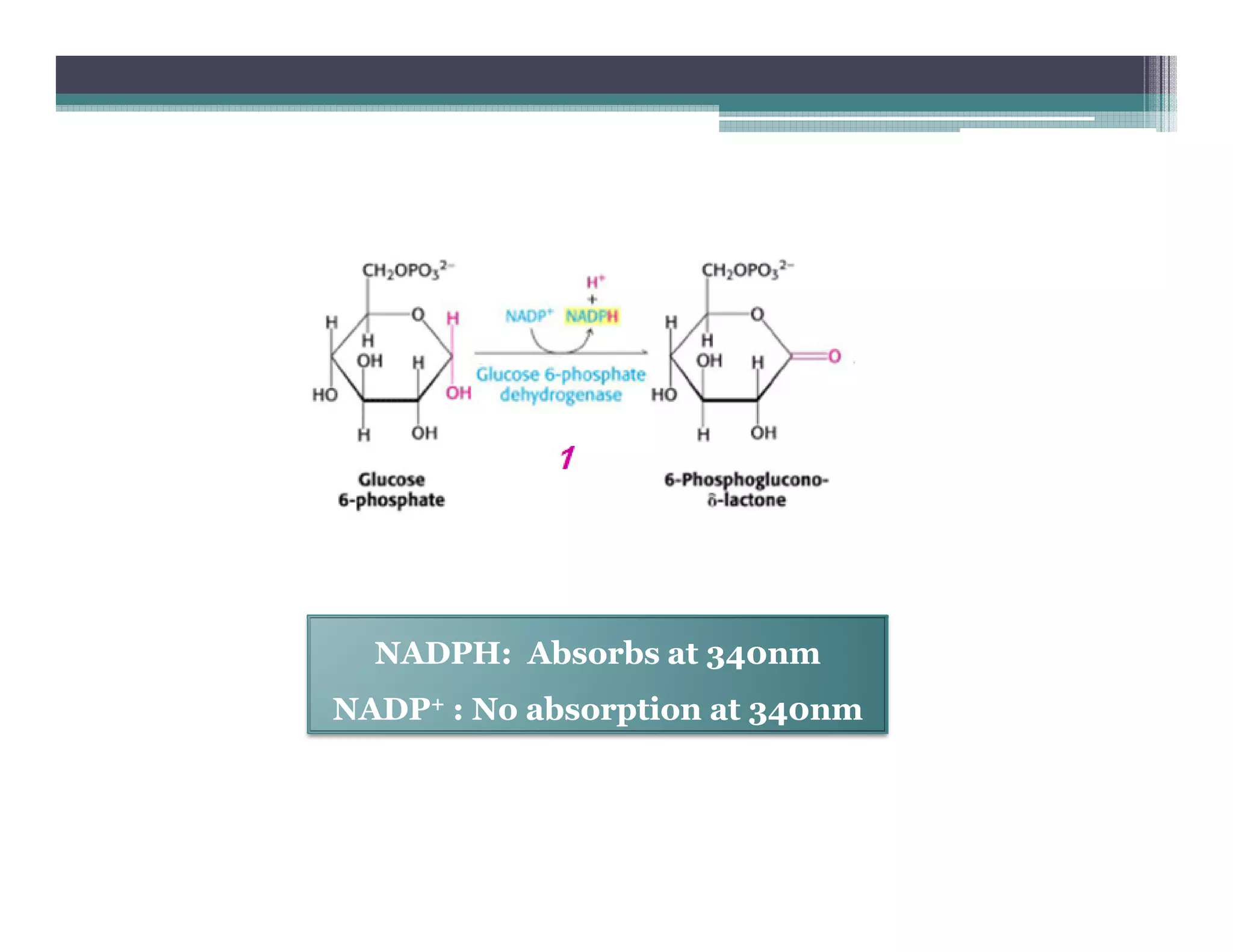
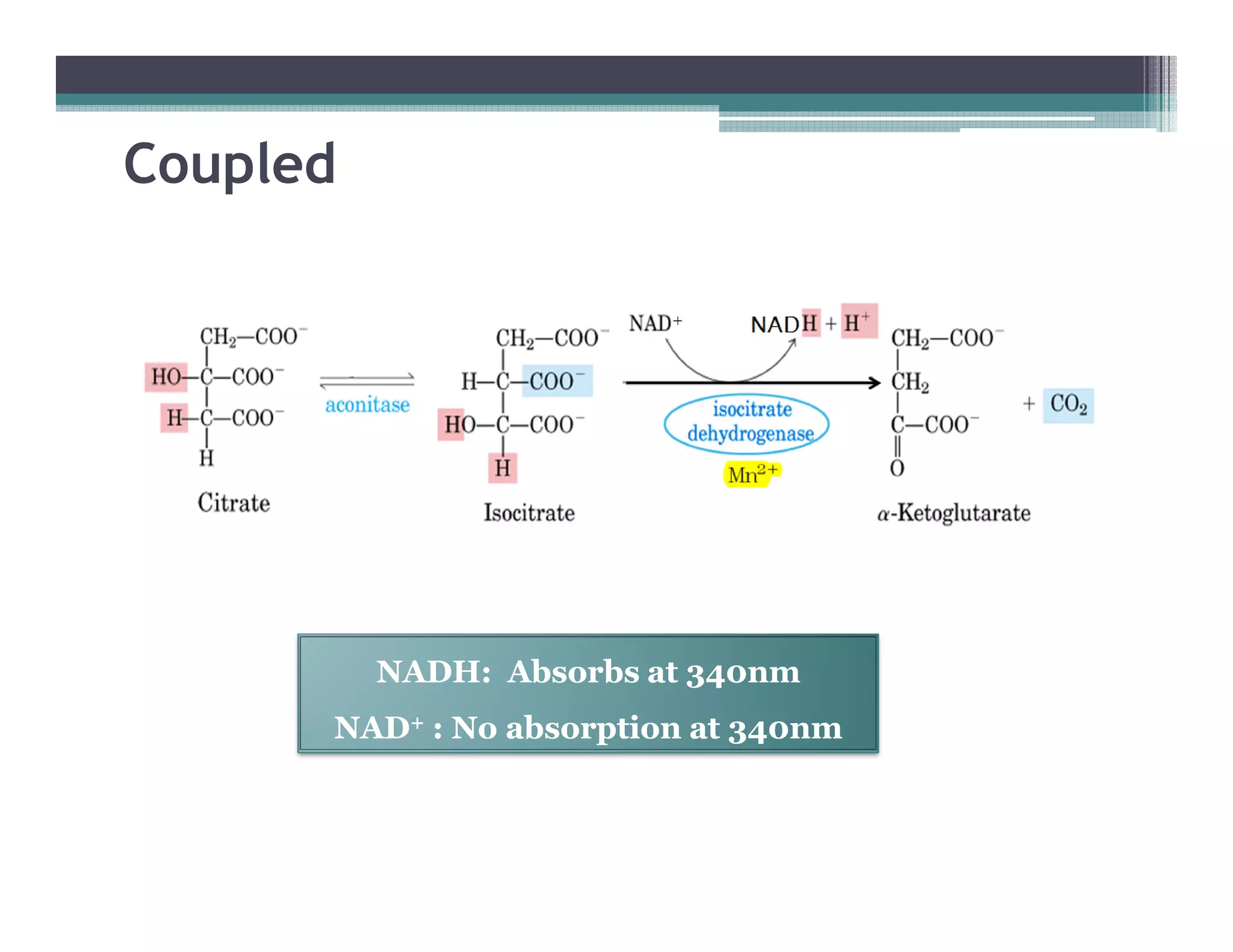
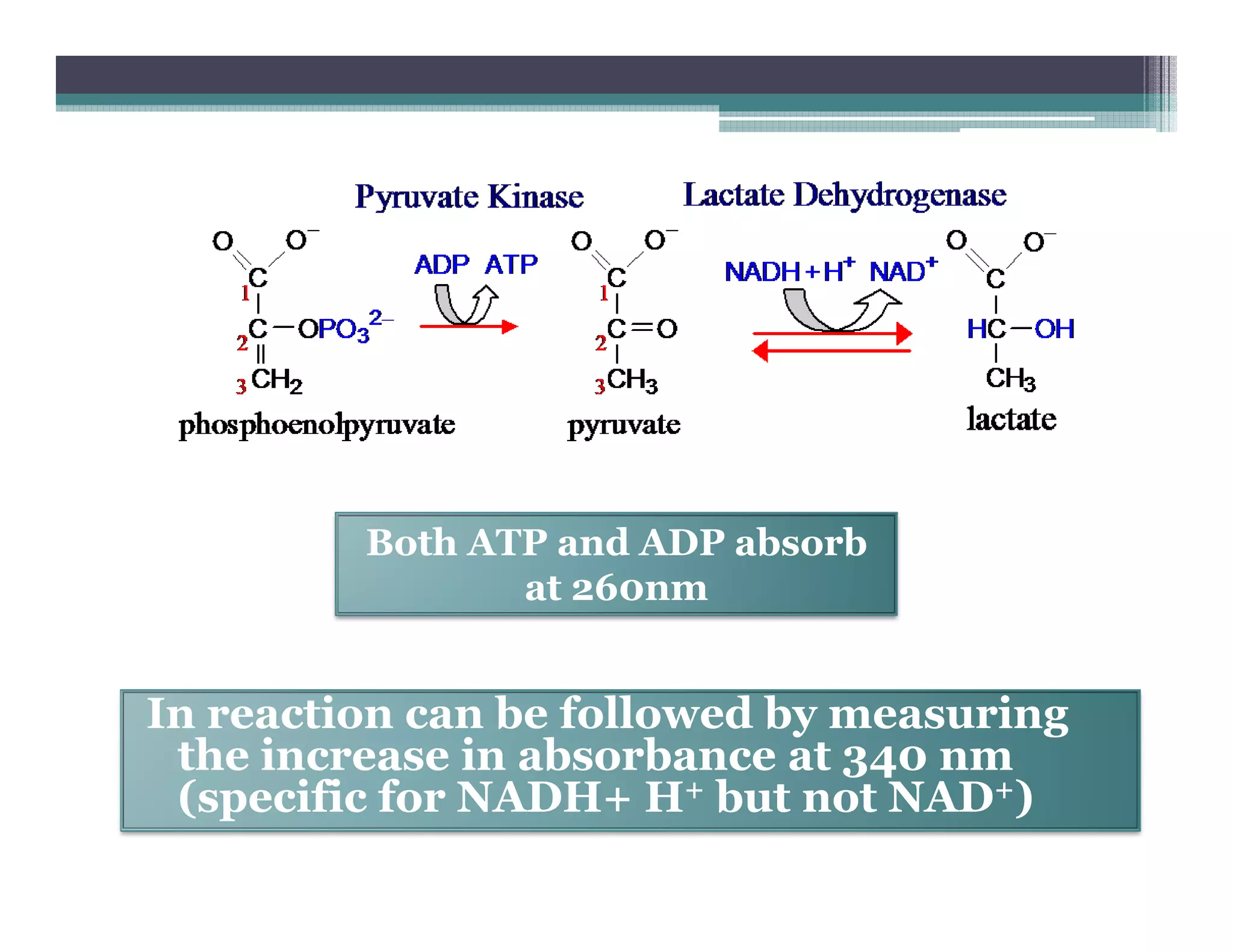
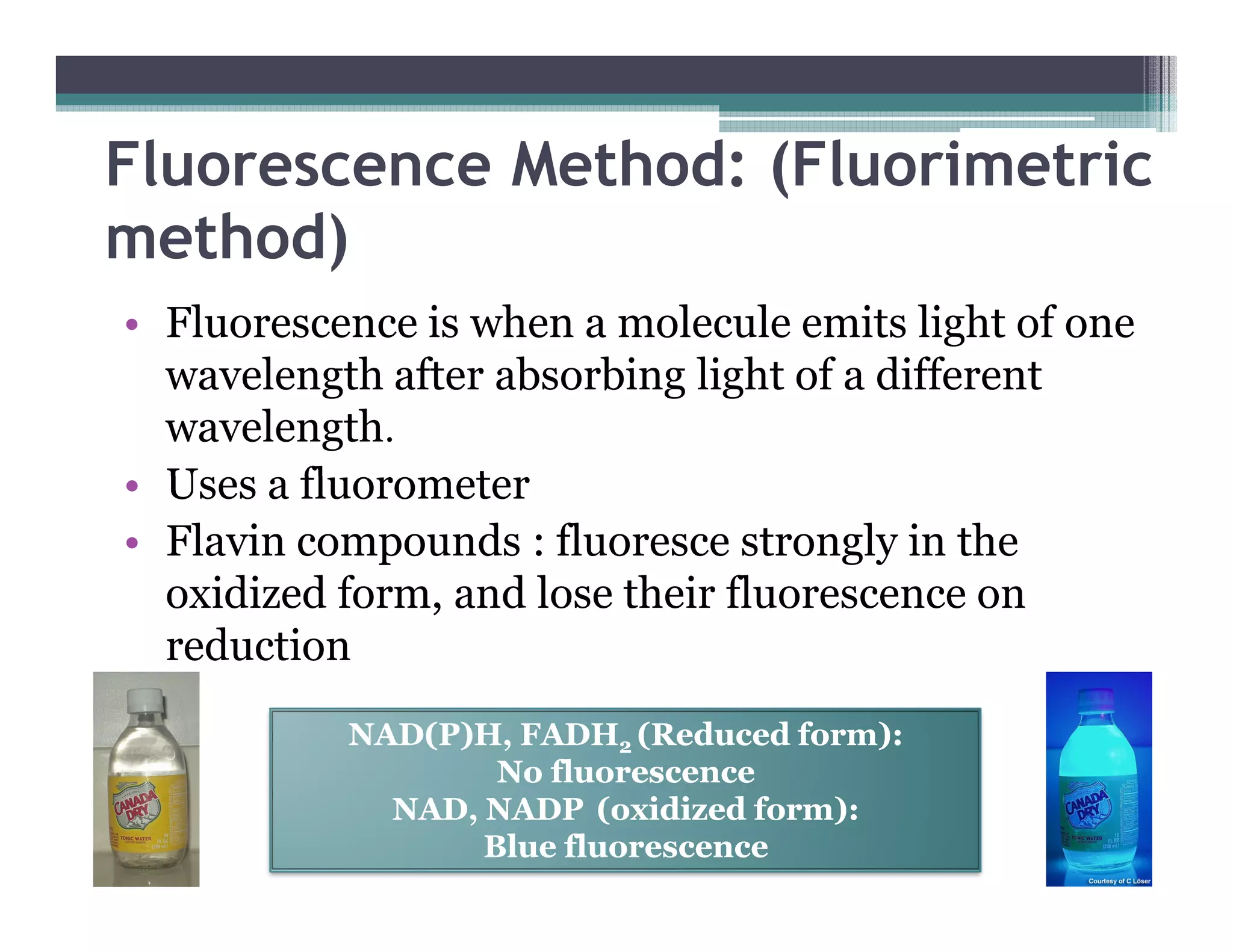
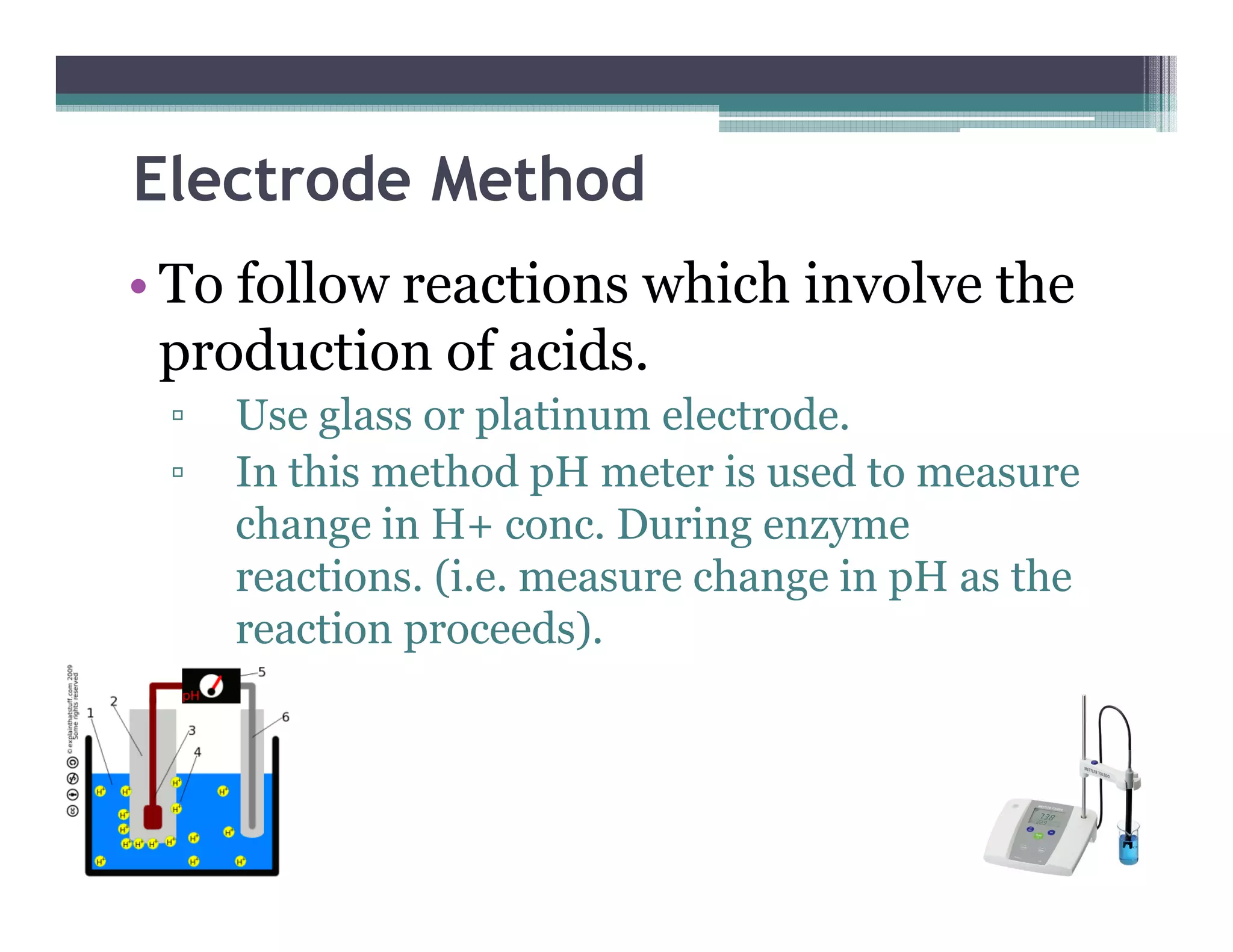
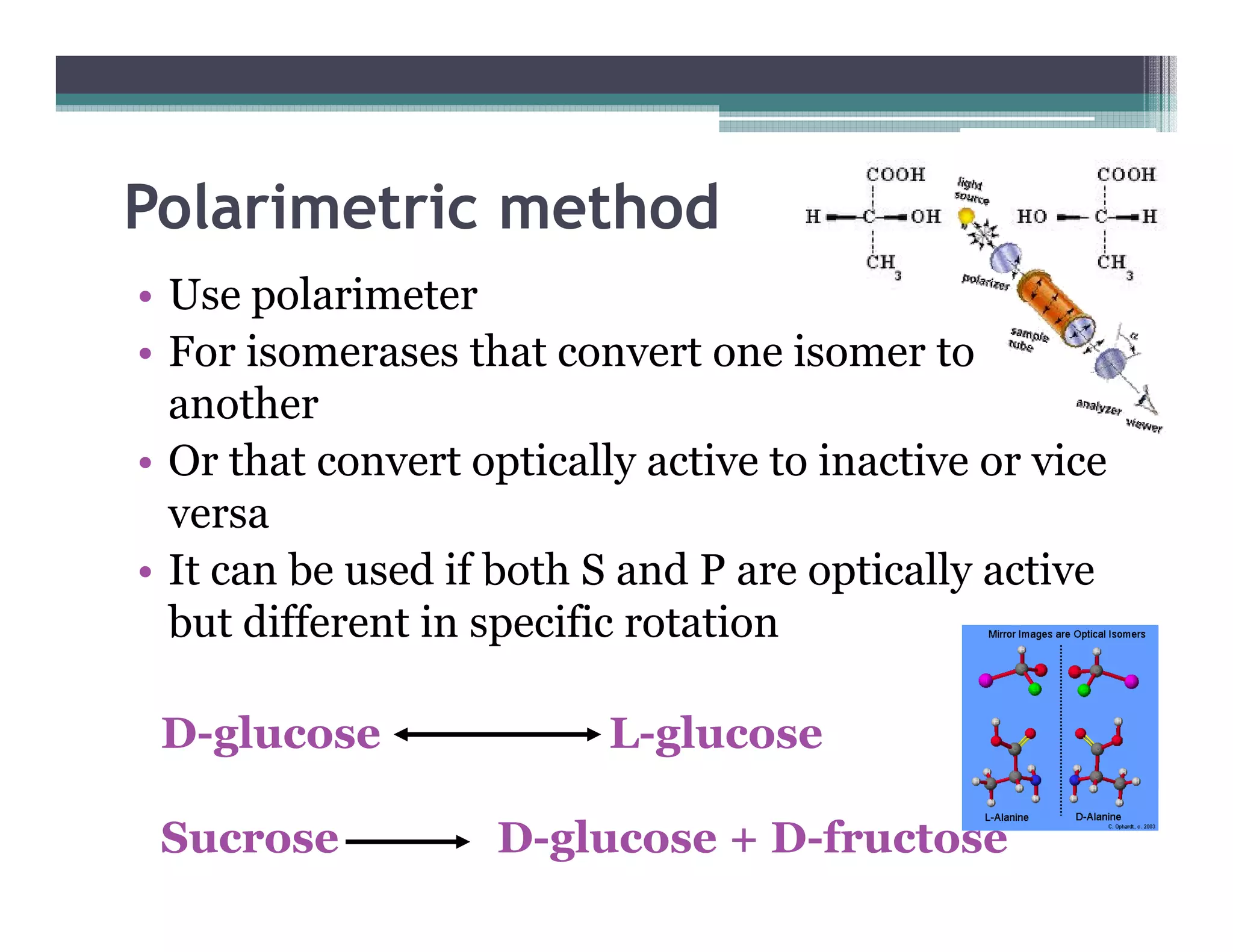
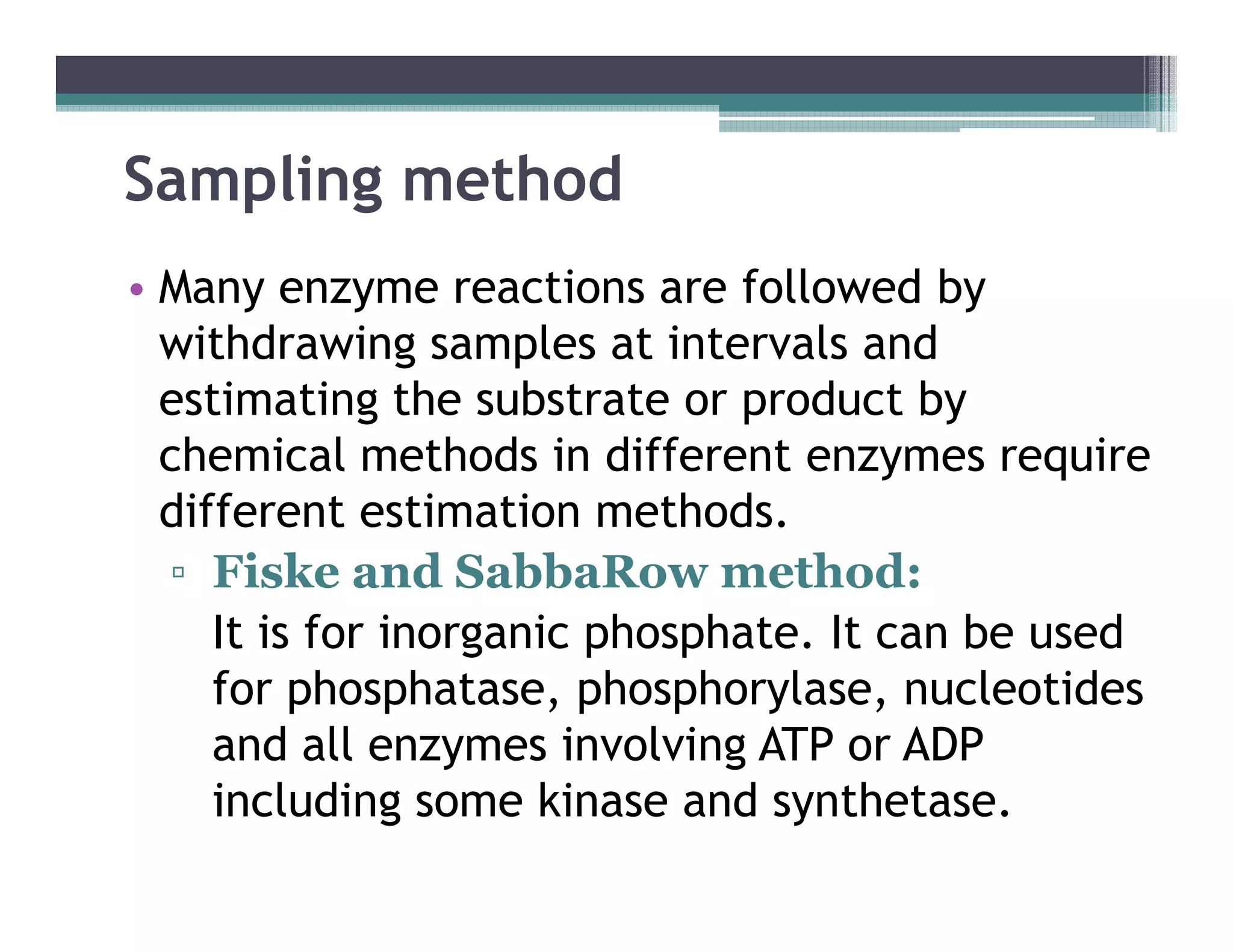
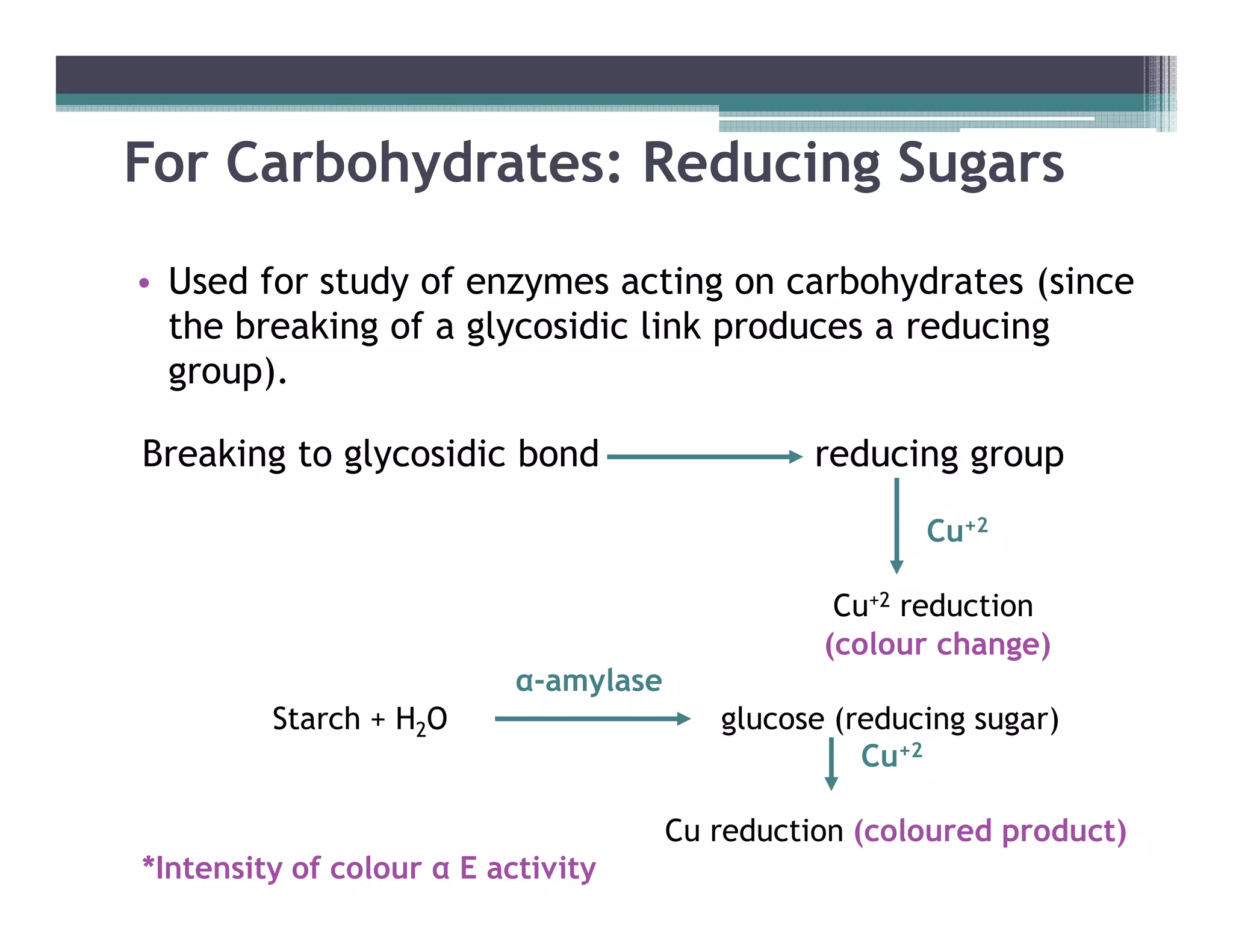
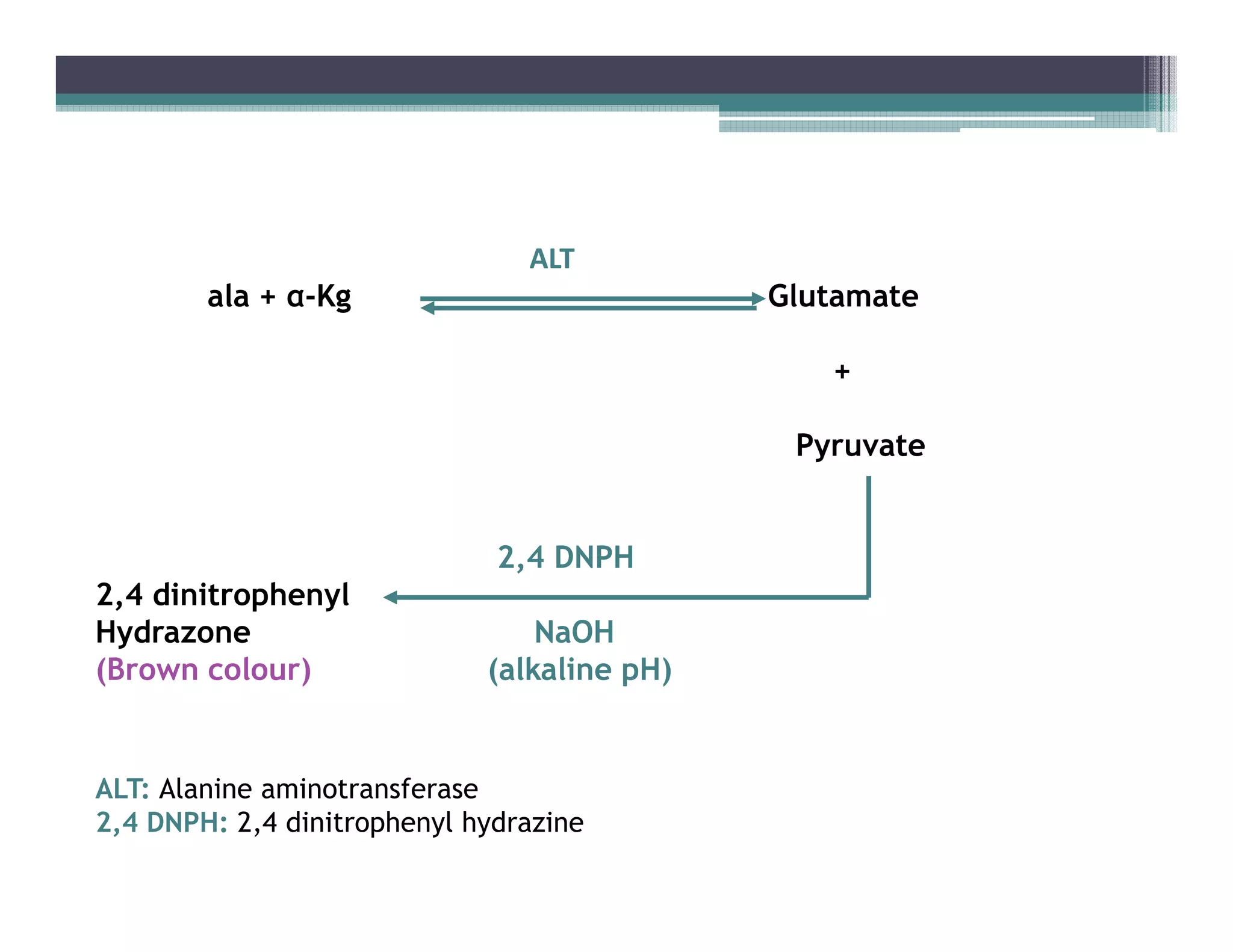
This document outlines various enzyme assay methods for measuring enzyme activity, emphasizing the importance of determining reaction velocity by tracking substrate conversion to product. It details methods such as spectrophotometric, fluorescence, manometric, electrode, polarimetric, and sampling methods, each suited for specific types of reactions or conditions. The document highlights the advantages and technical specifics of techniques, thereby aiding in the selection of appropriate methods based on enzyme characteristics.