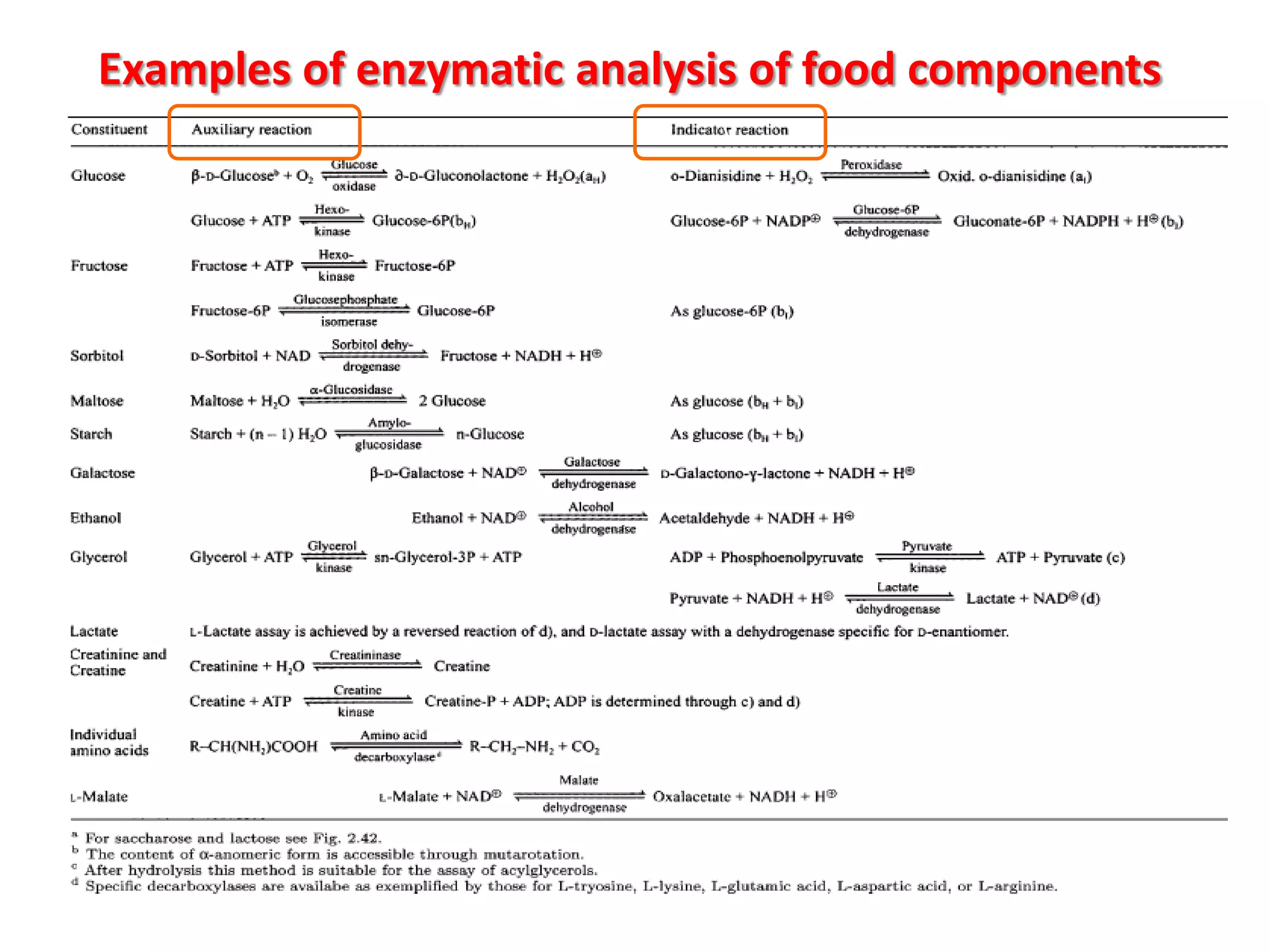
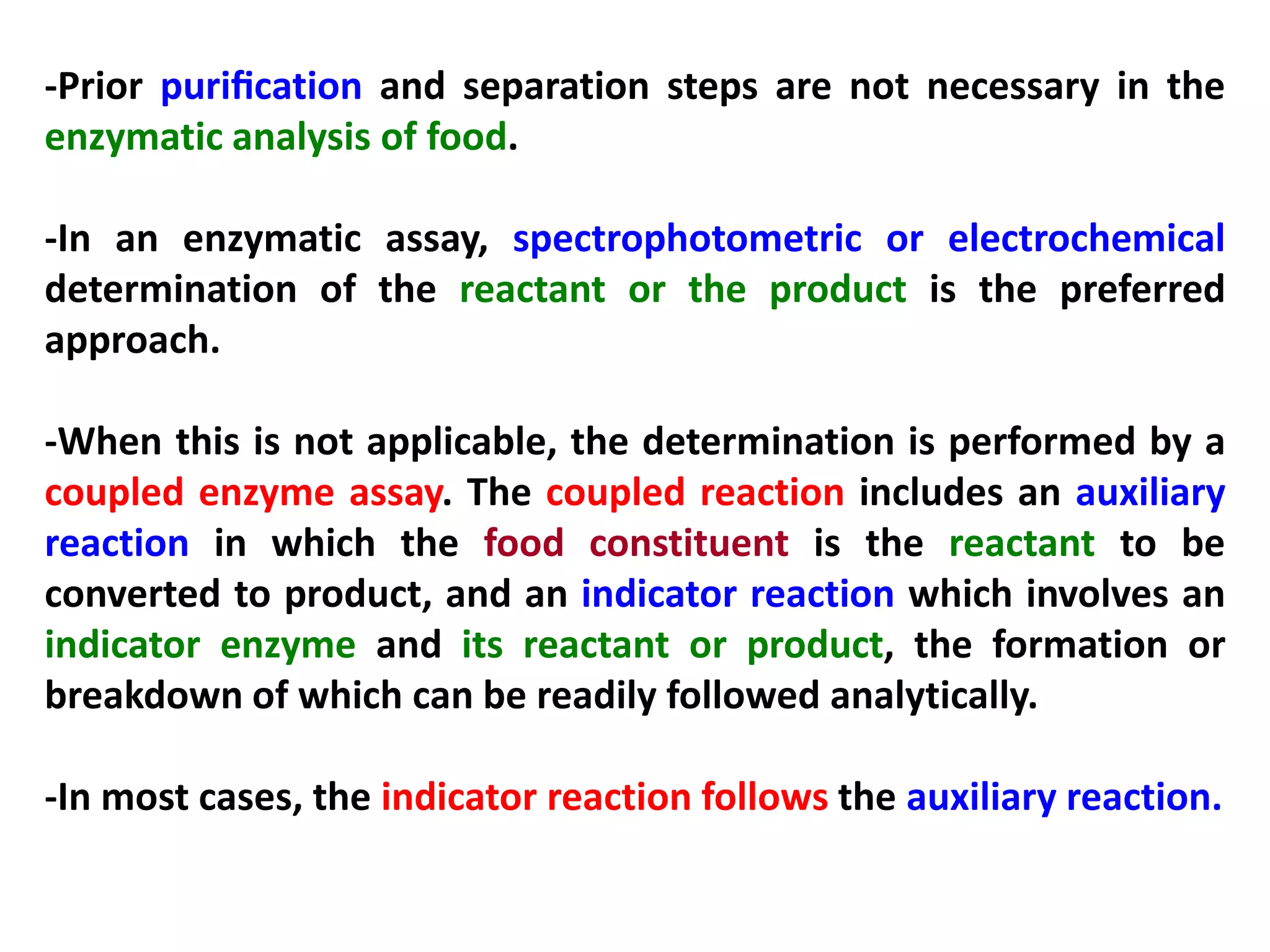
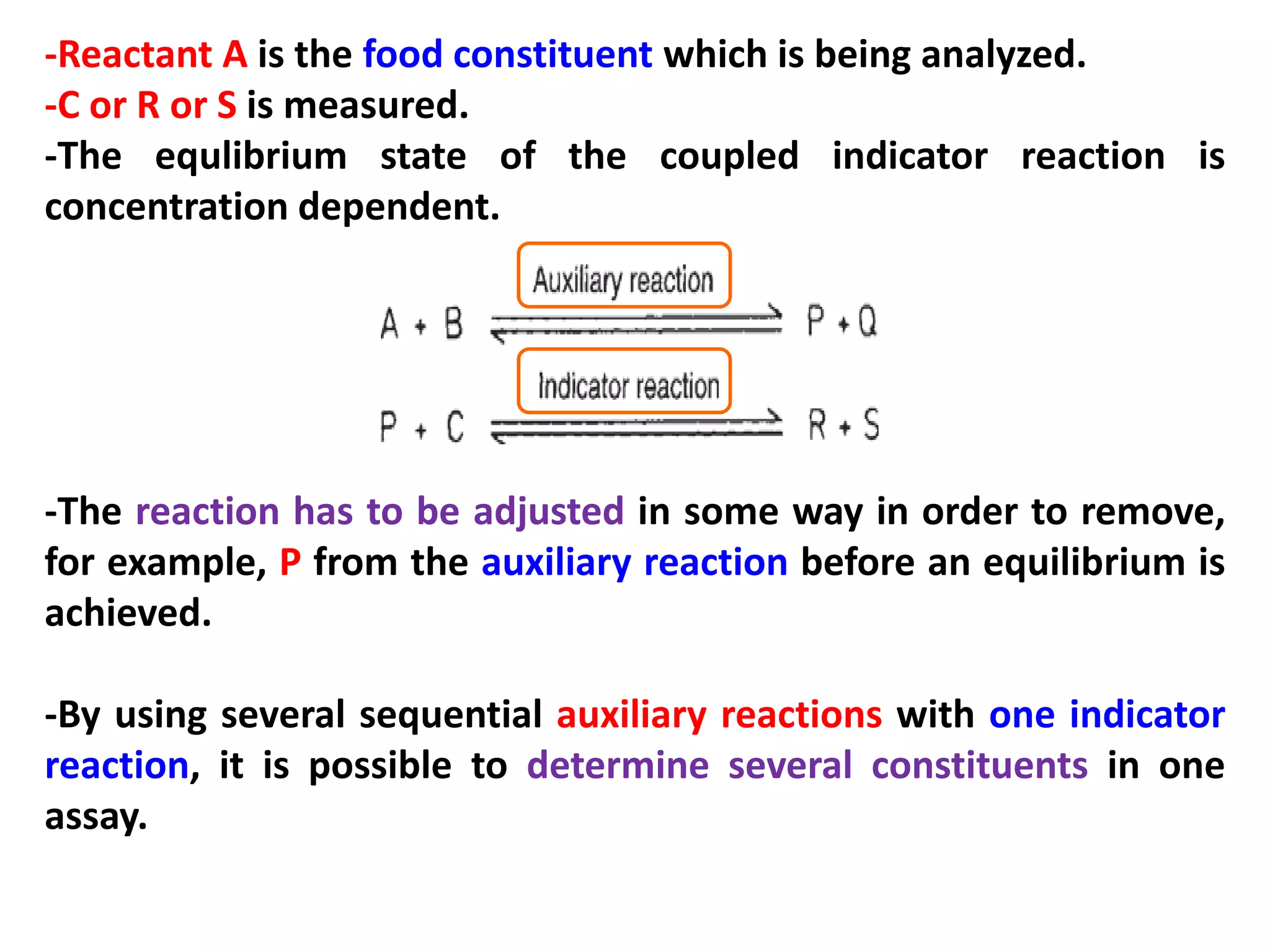
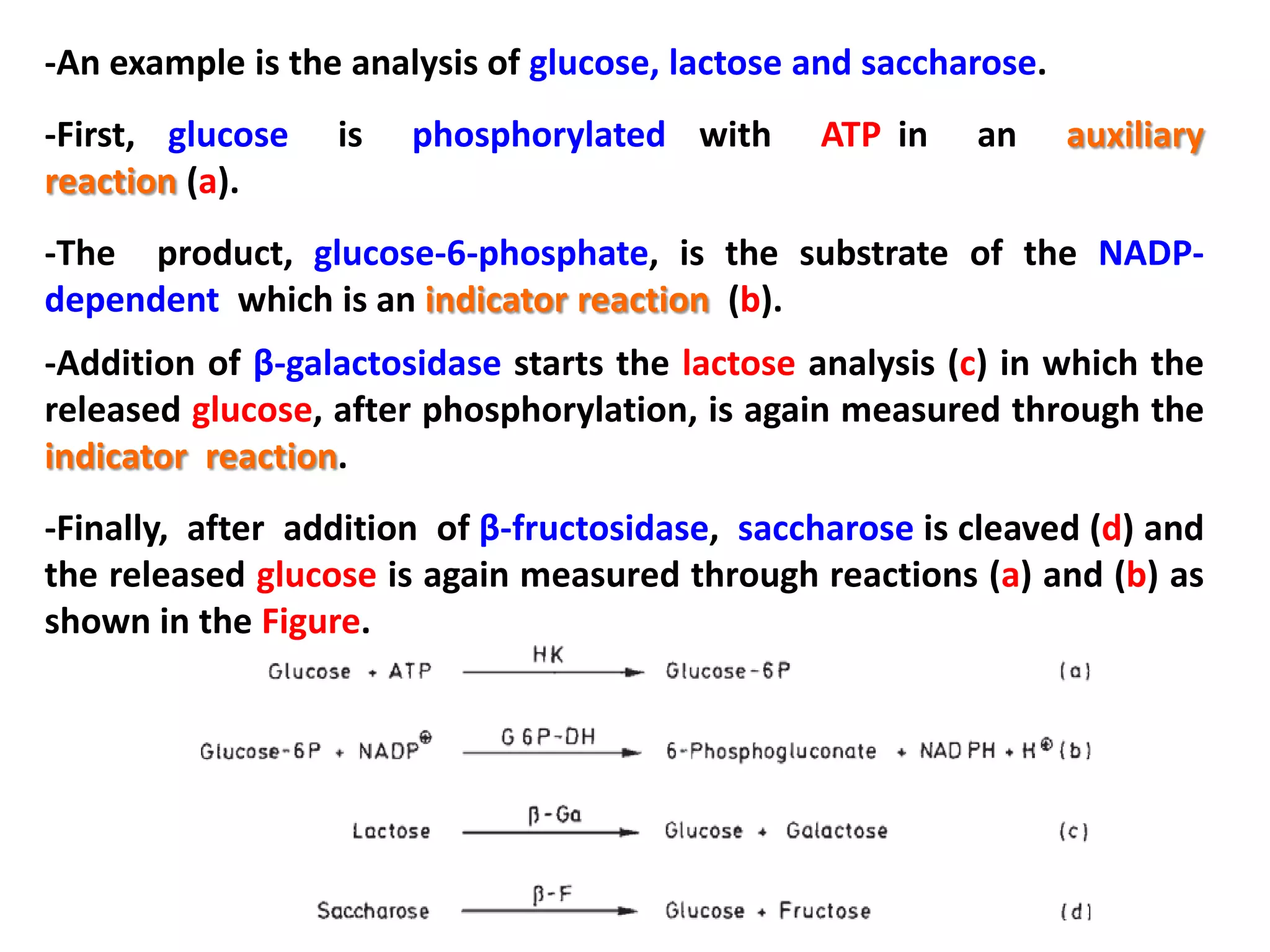
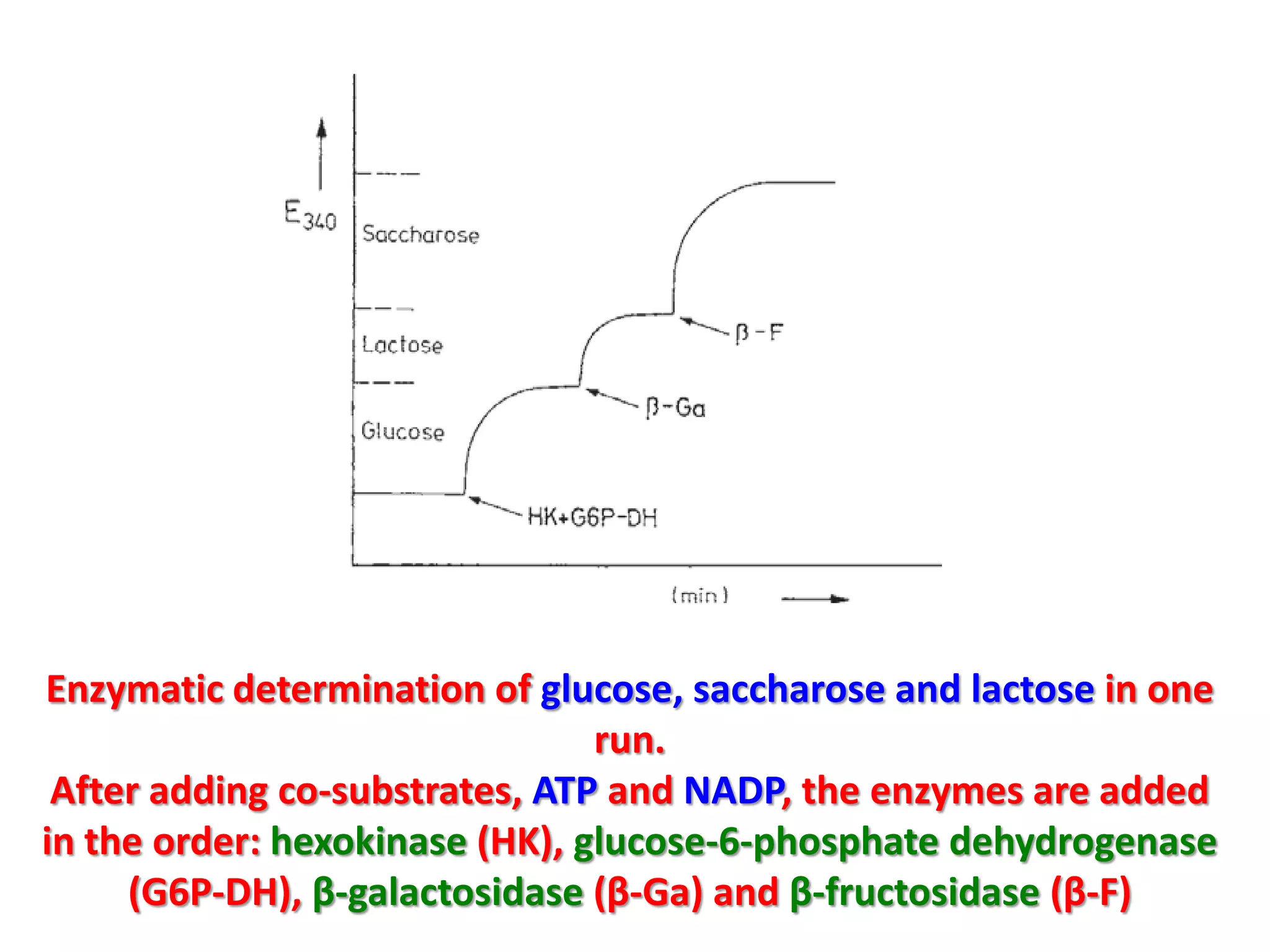
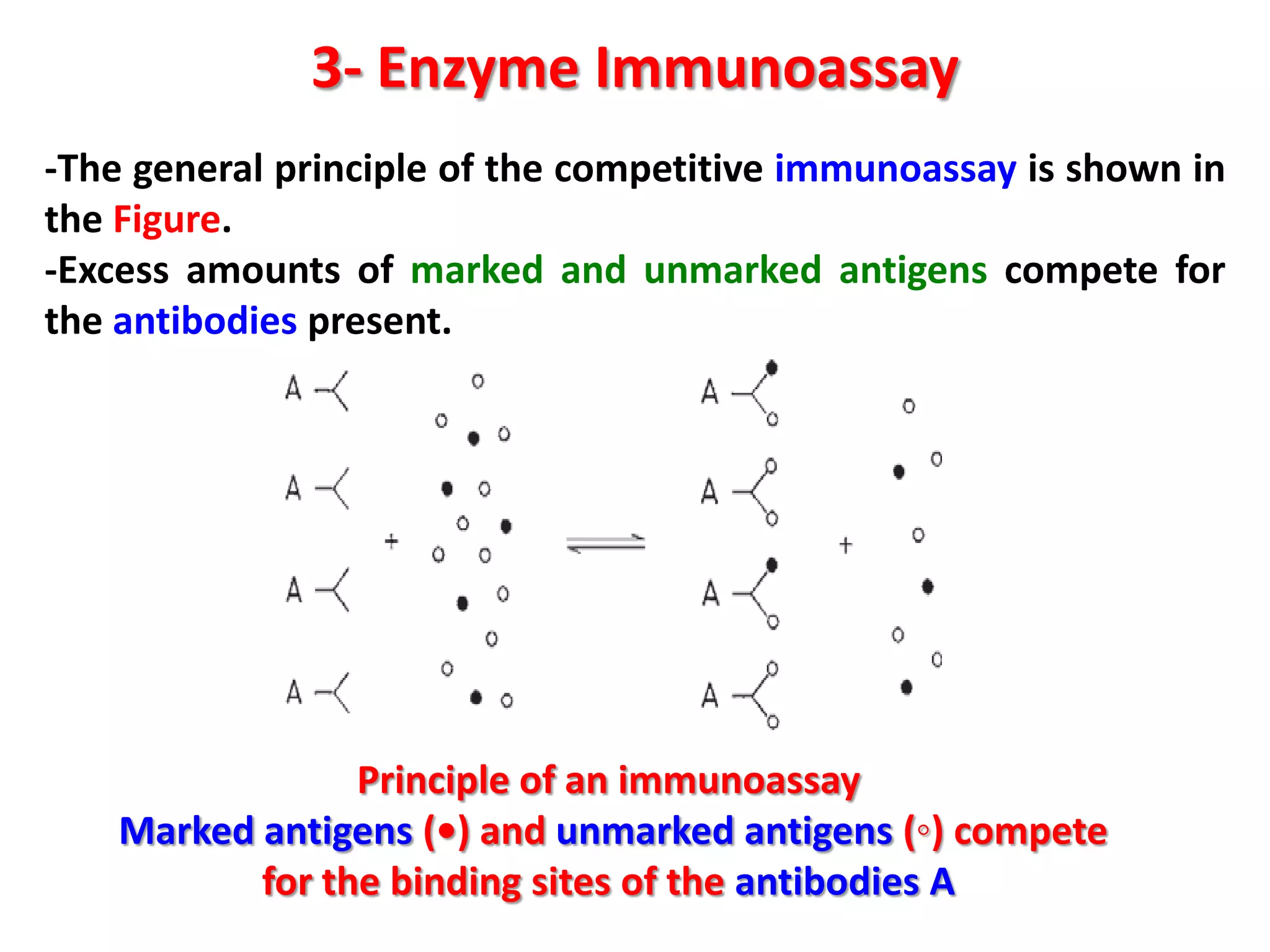
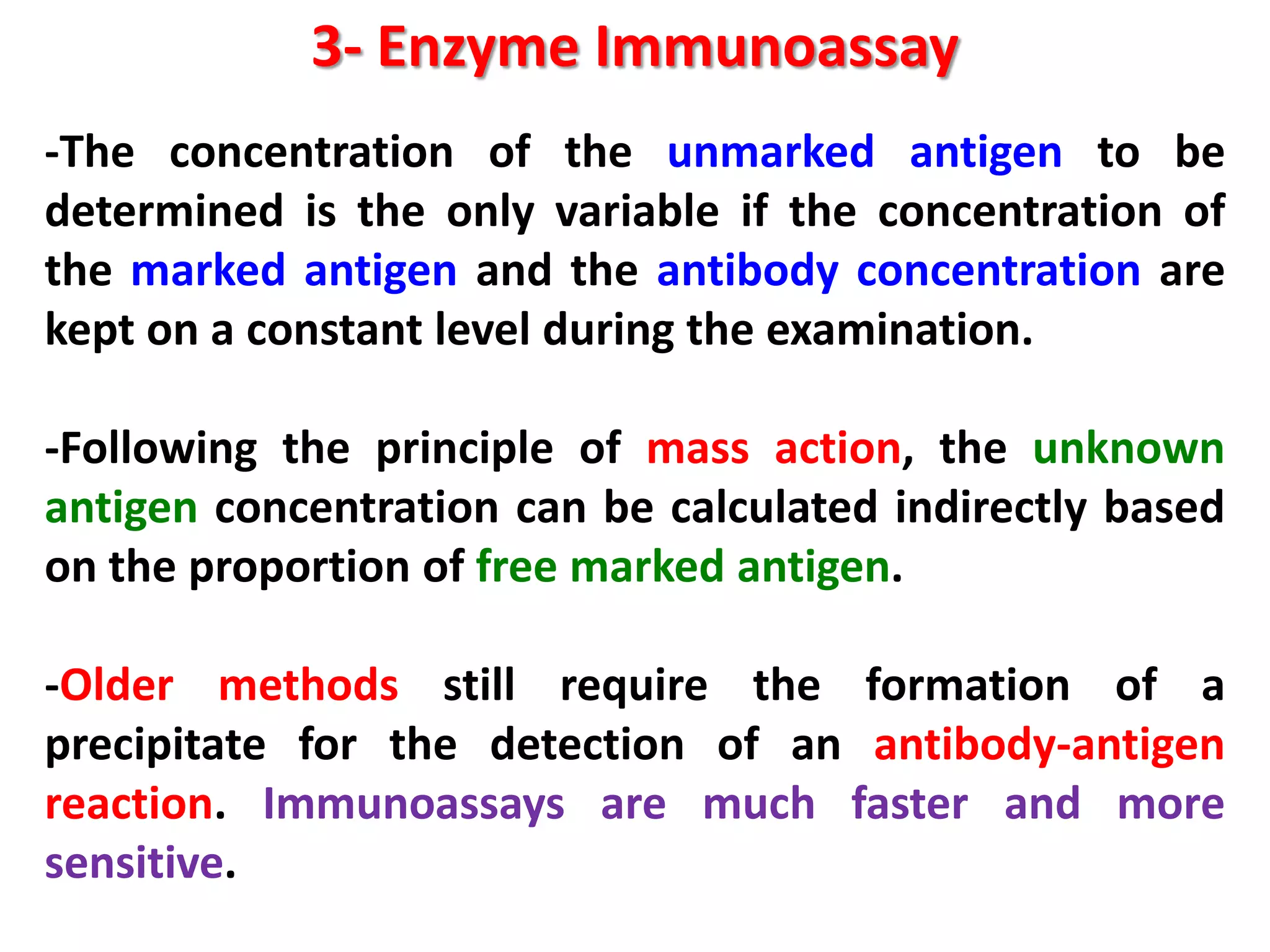
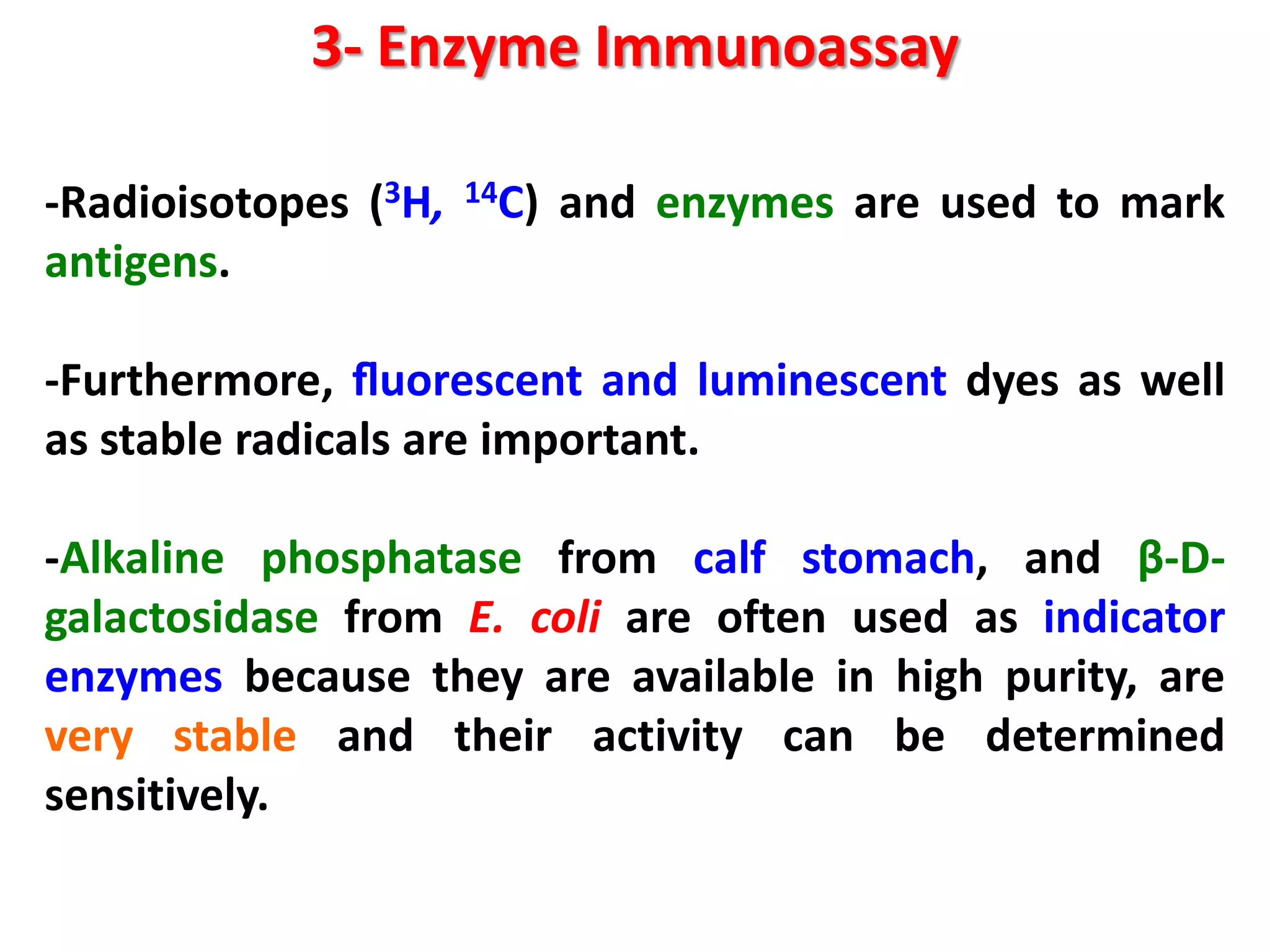
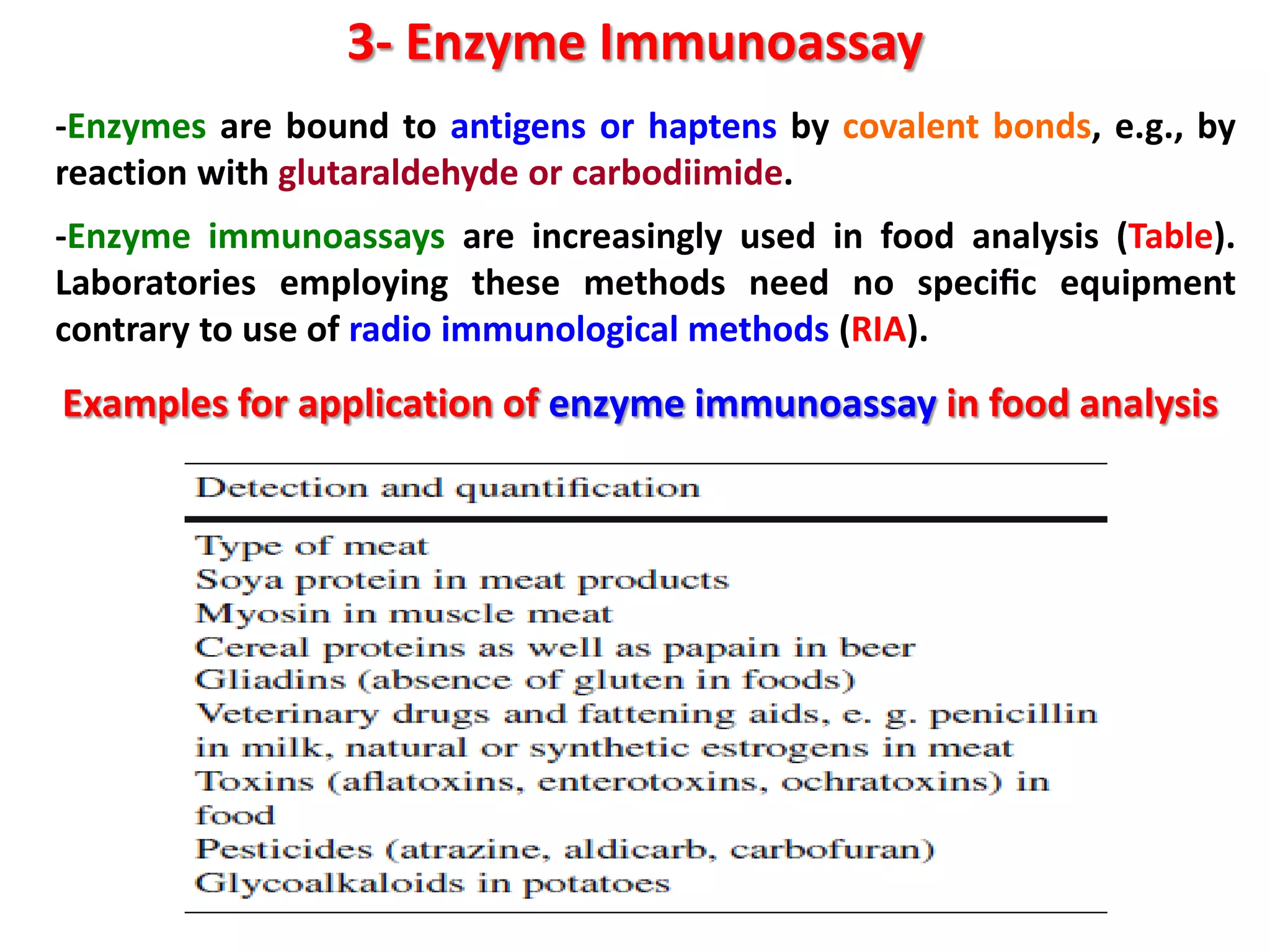
This document discusses various enzymatic analytical methods used for food analysis, including substrate determination, enzyme activity determination, enzyme immunoassay, and polymerase chain reaction. Specifically, it covers:
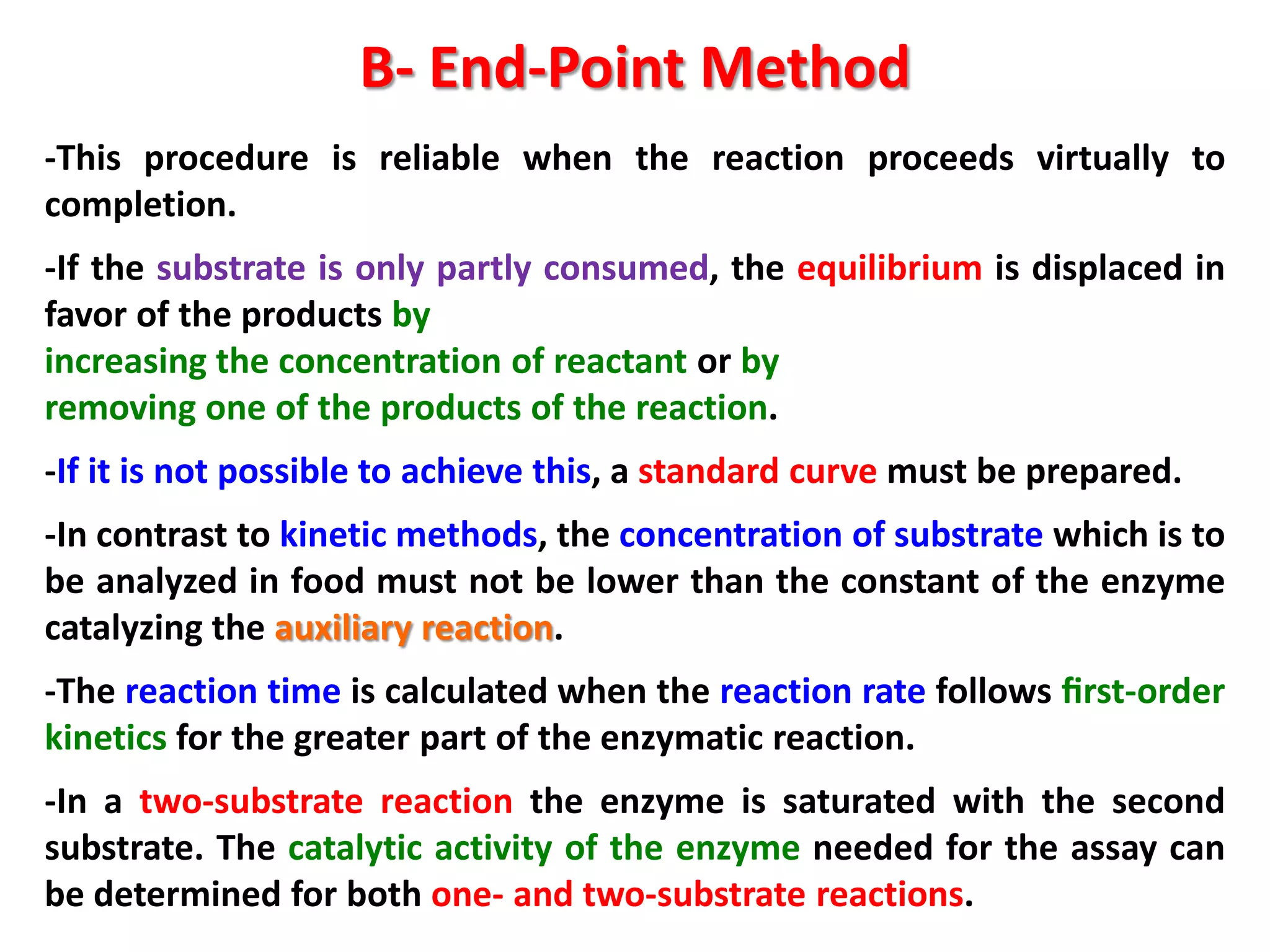
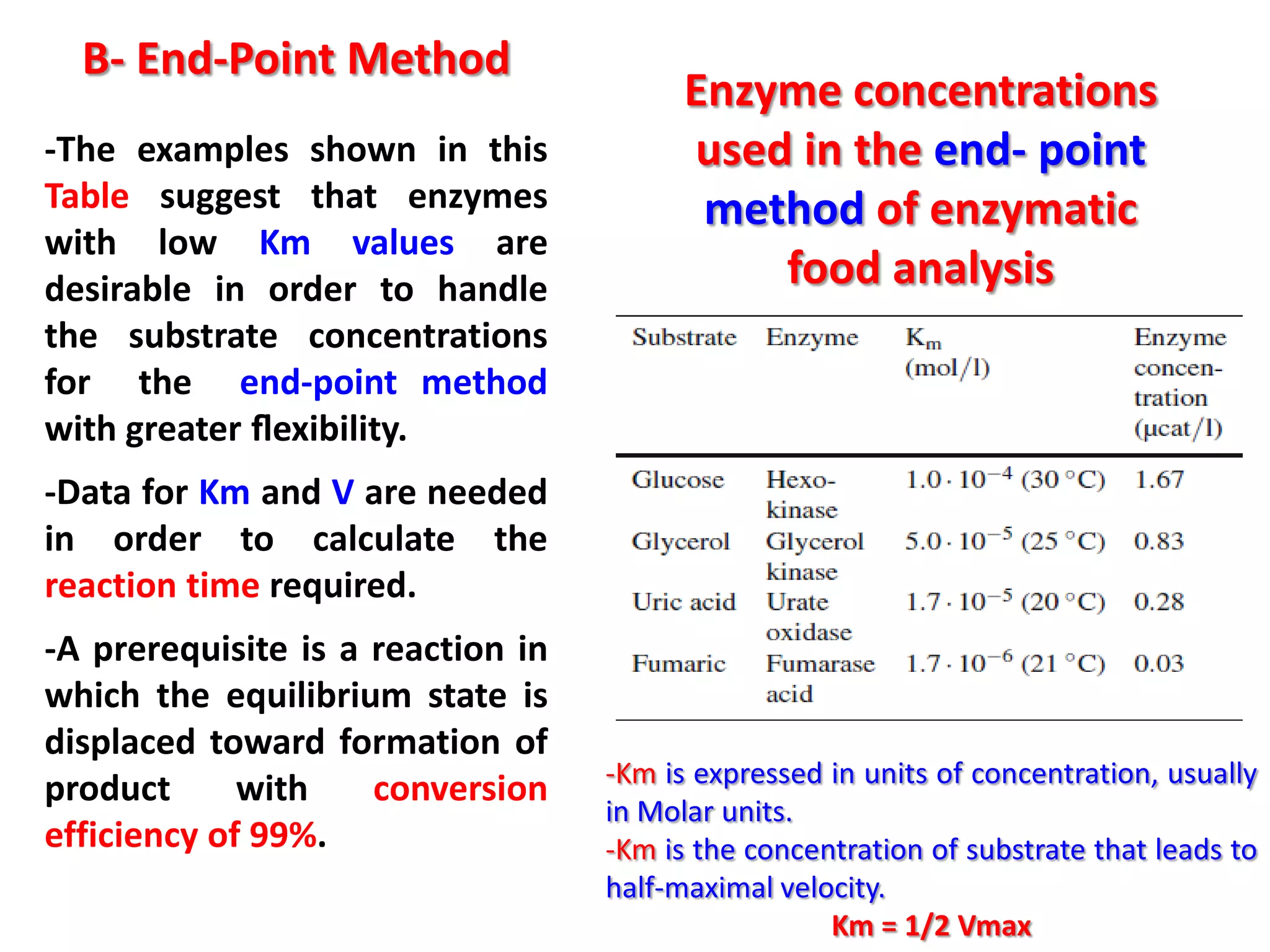
1) Substrate determination methods like end-point assays that use coupled enzyme reactions to measure food constituents.
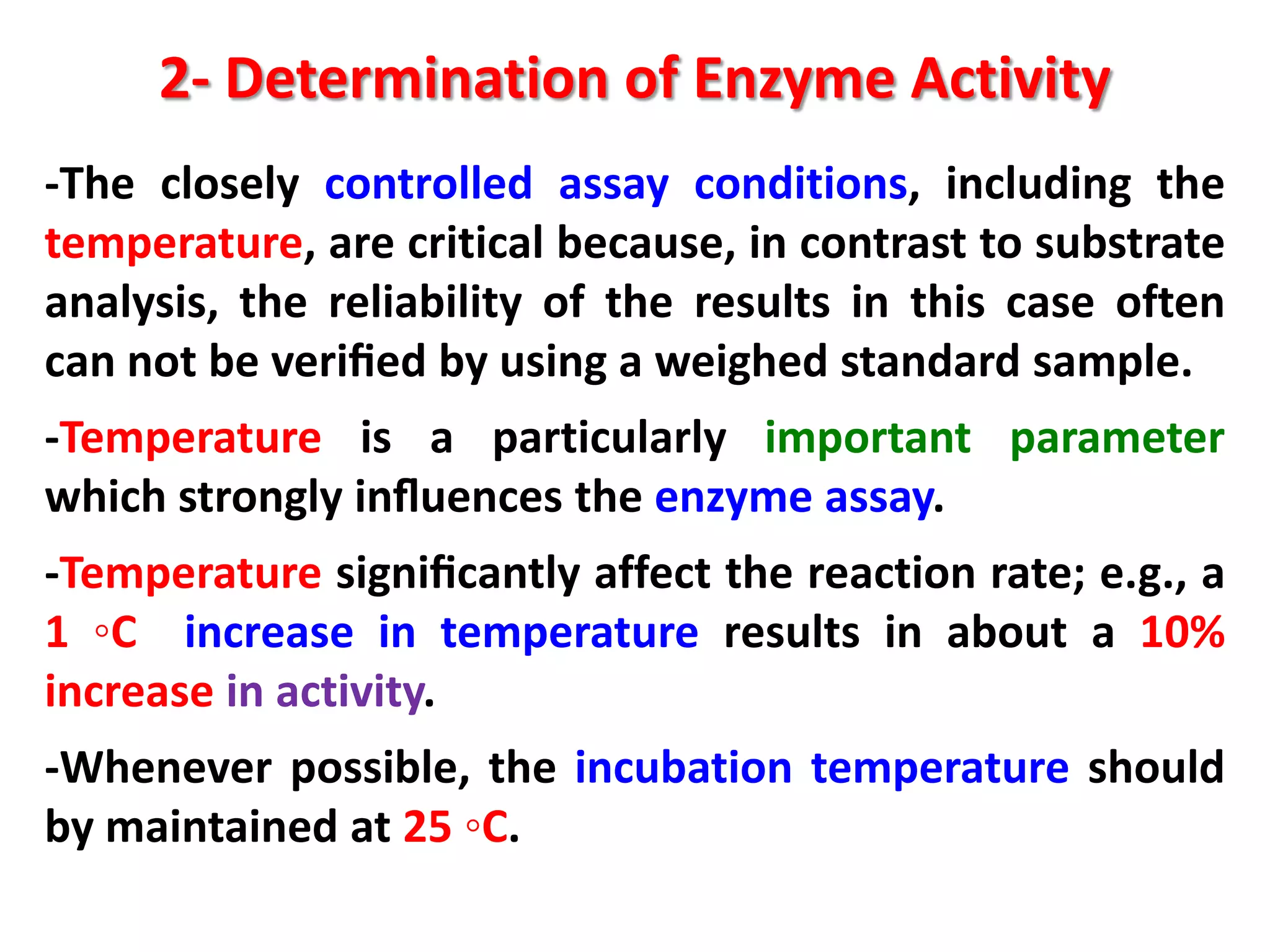
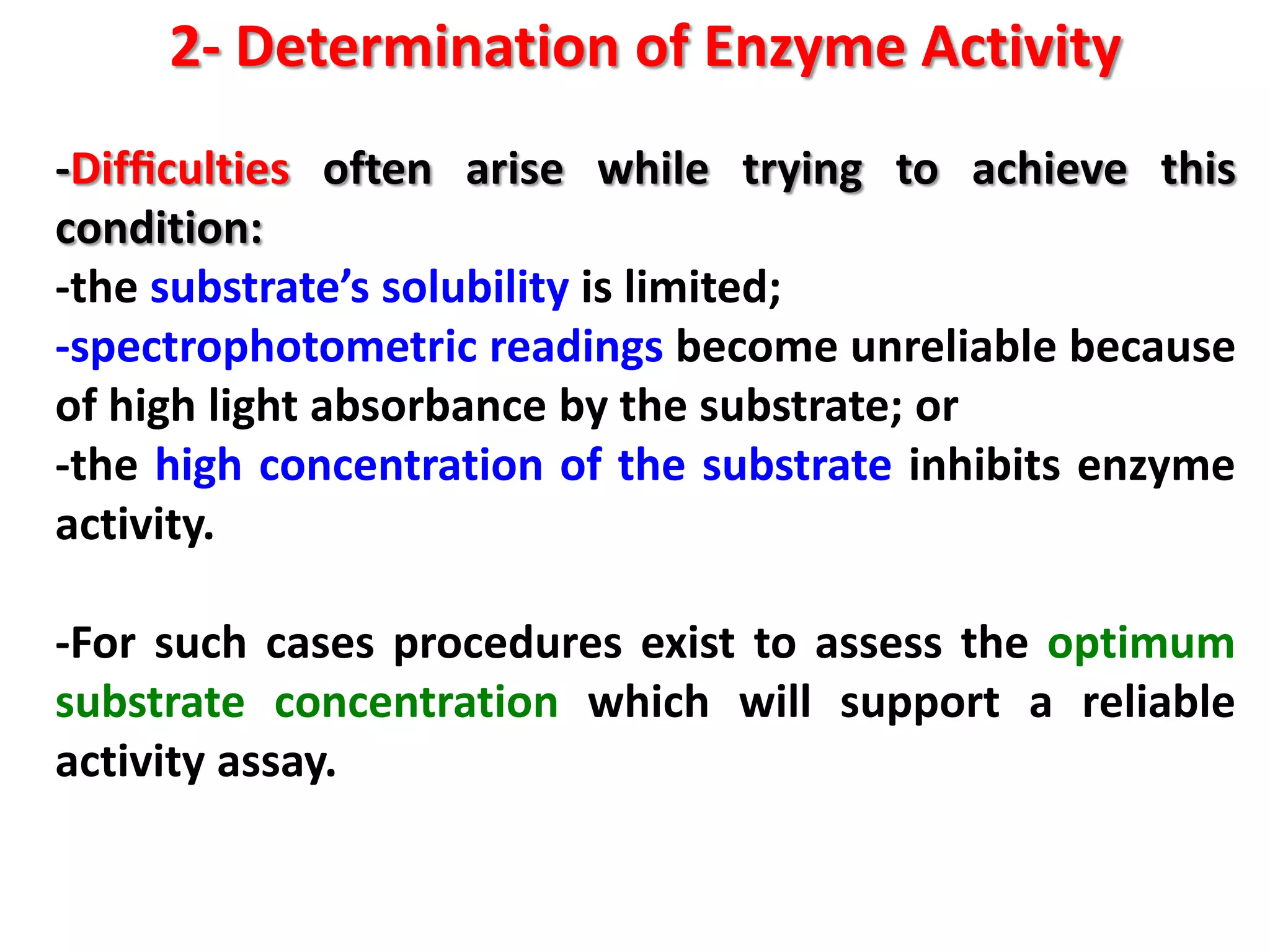
2) Determining enzyme activity in food to evaluate quality and optimize processes by measuring reaction rates under controlled conditions.
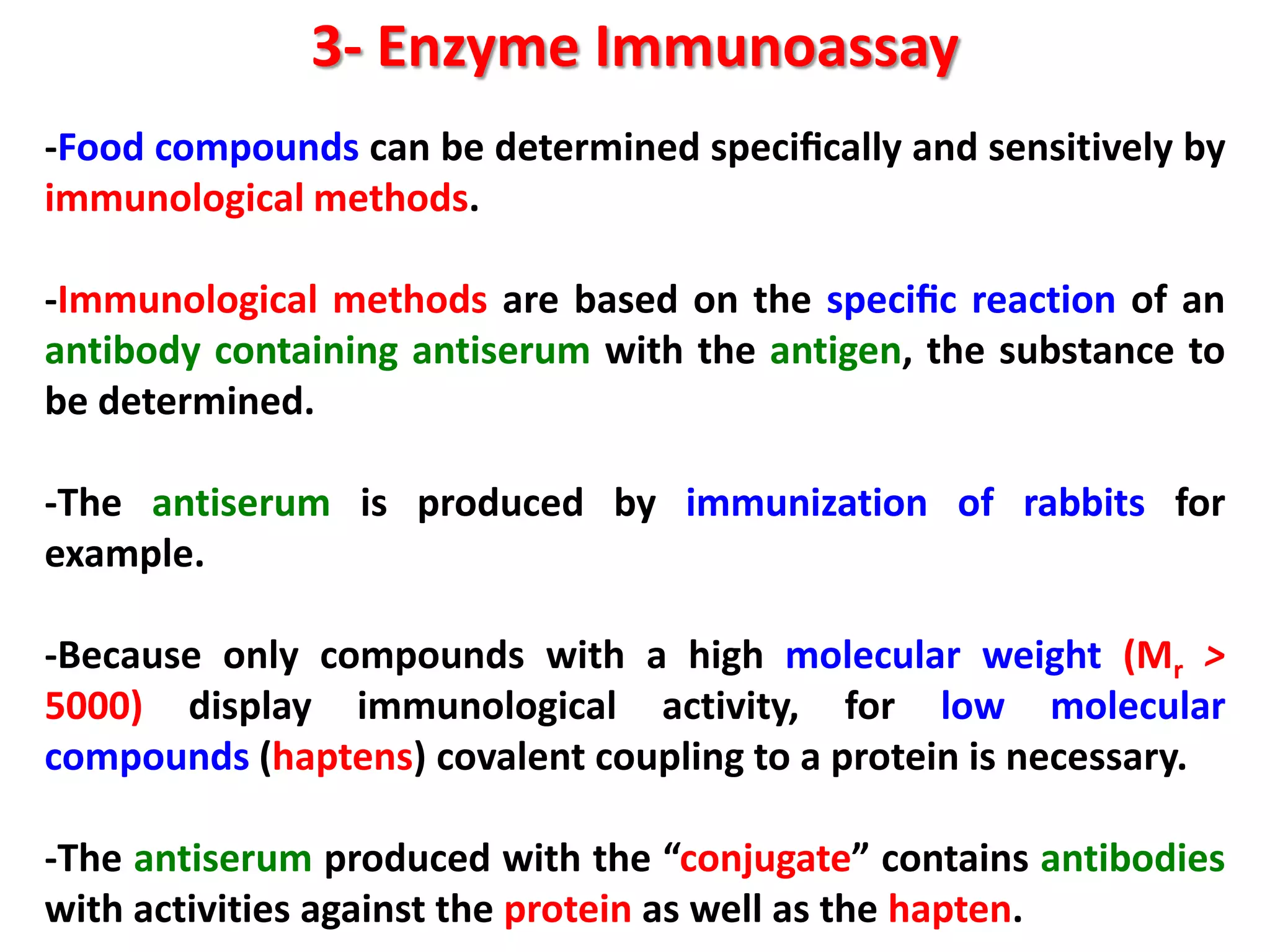
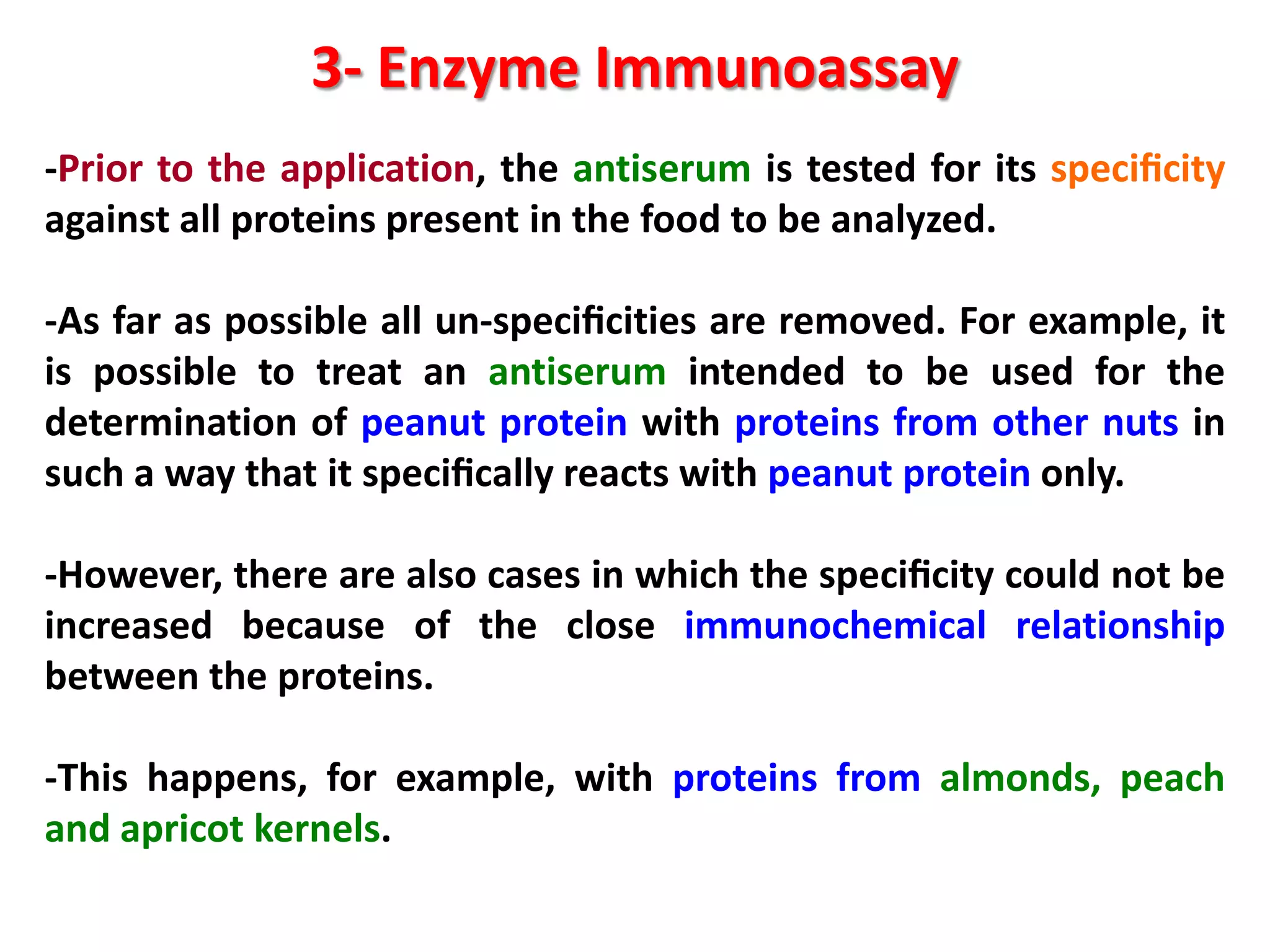
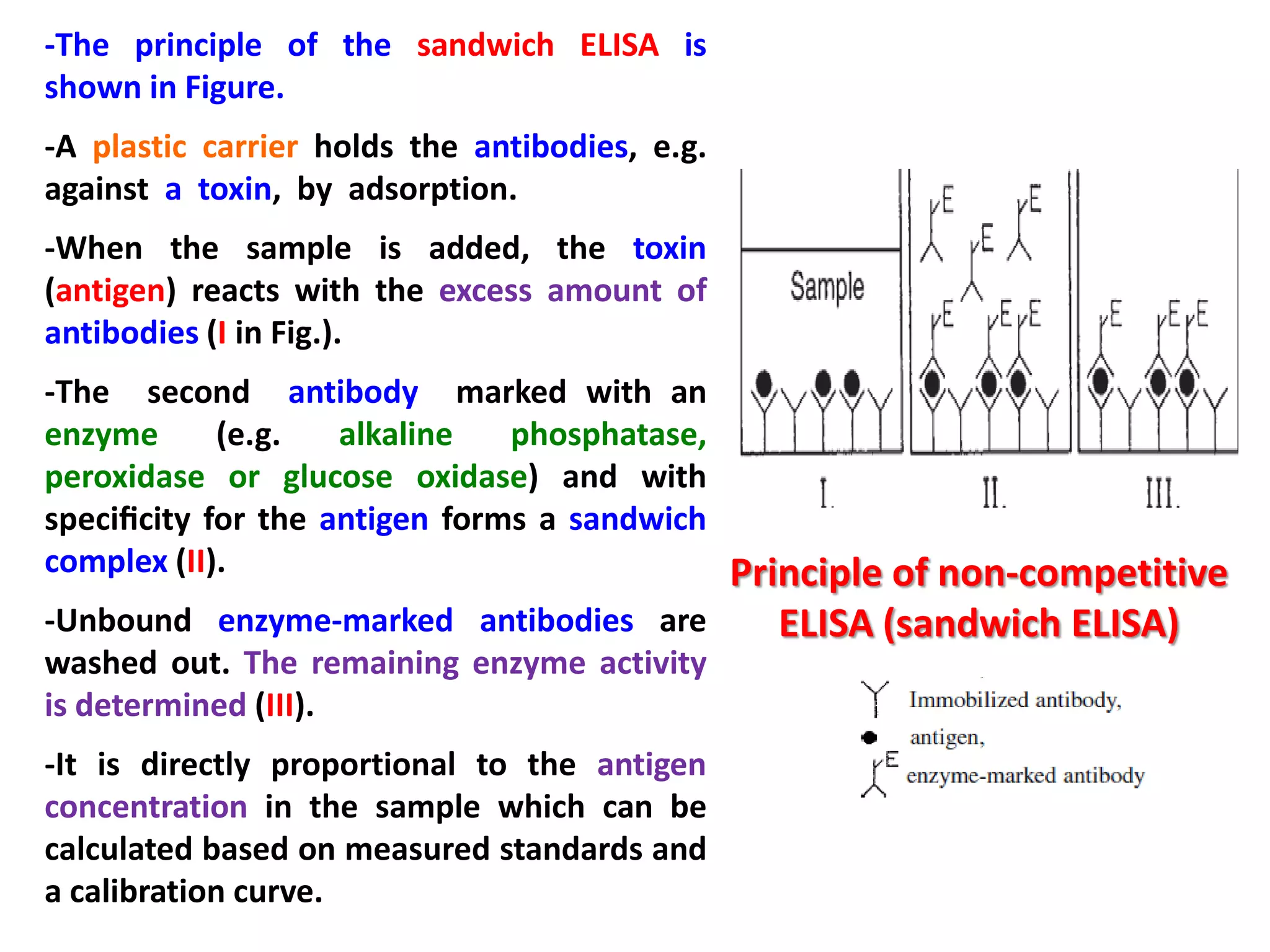
3) Enzyme immunoassays like ELISA that use antibody-antigen reactions and enzyme-labeled antigens to detect food compounds.
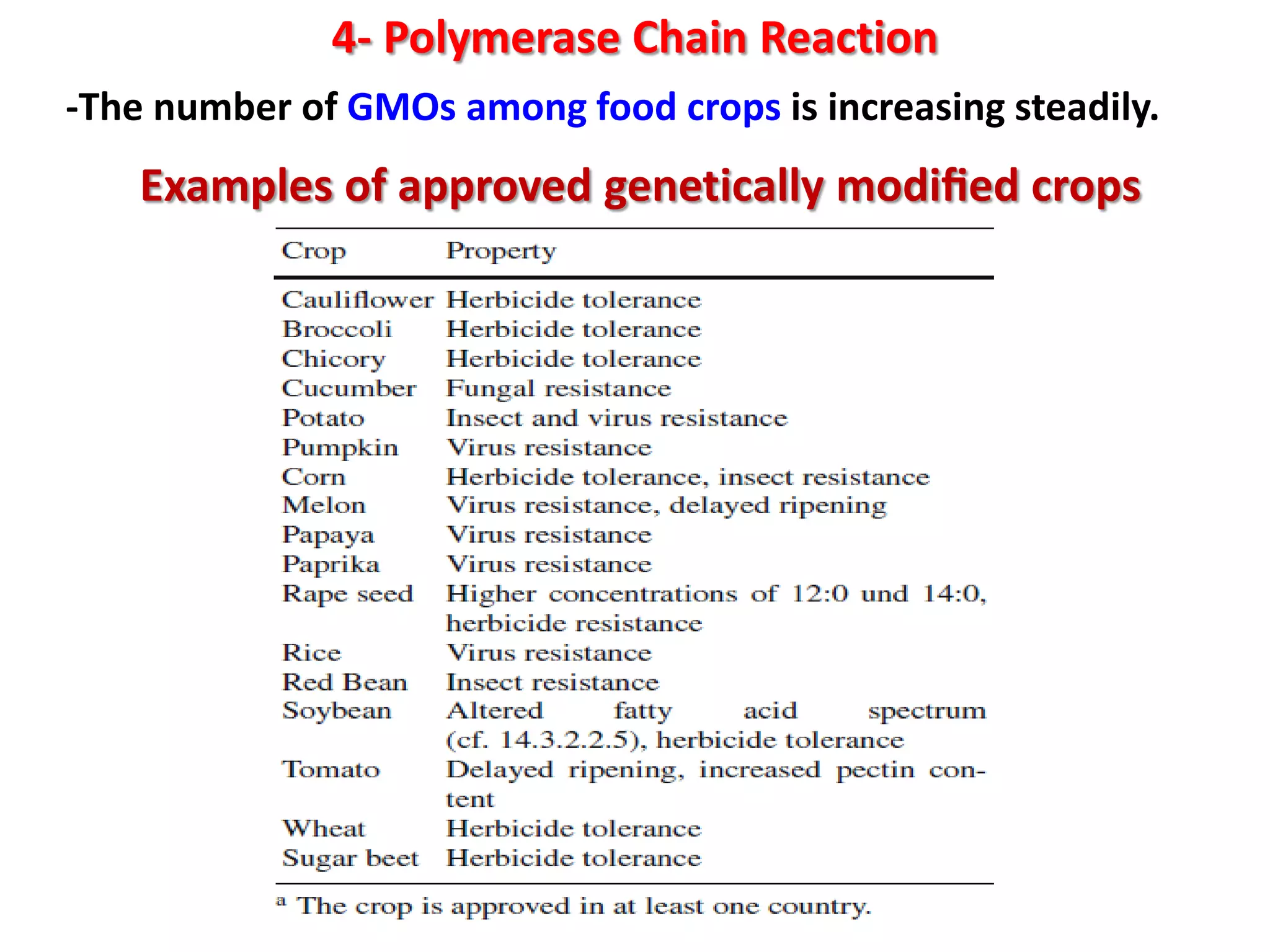
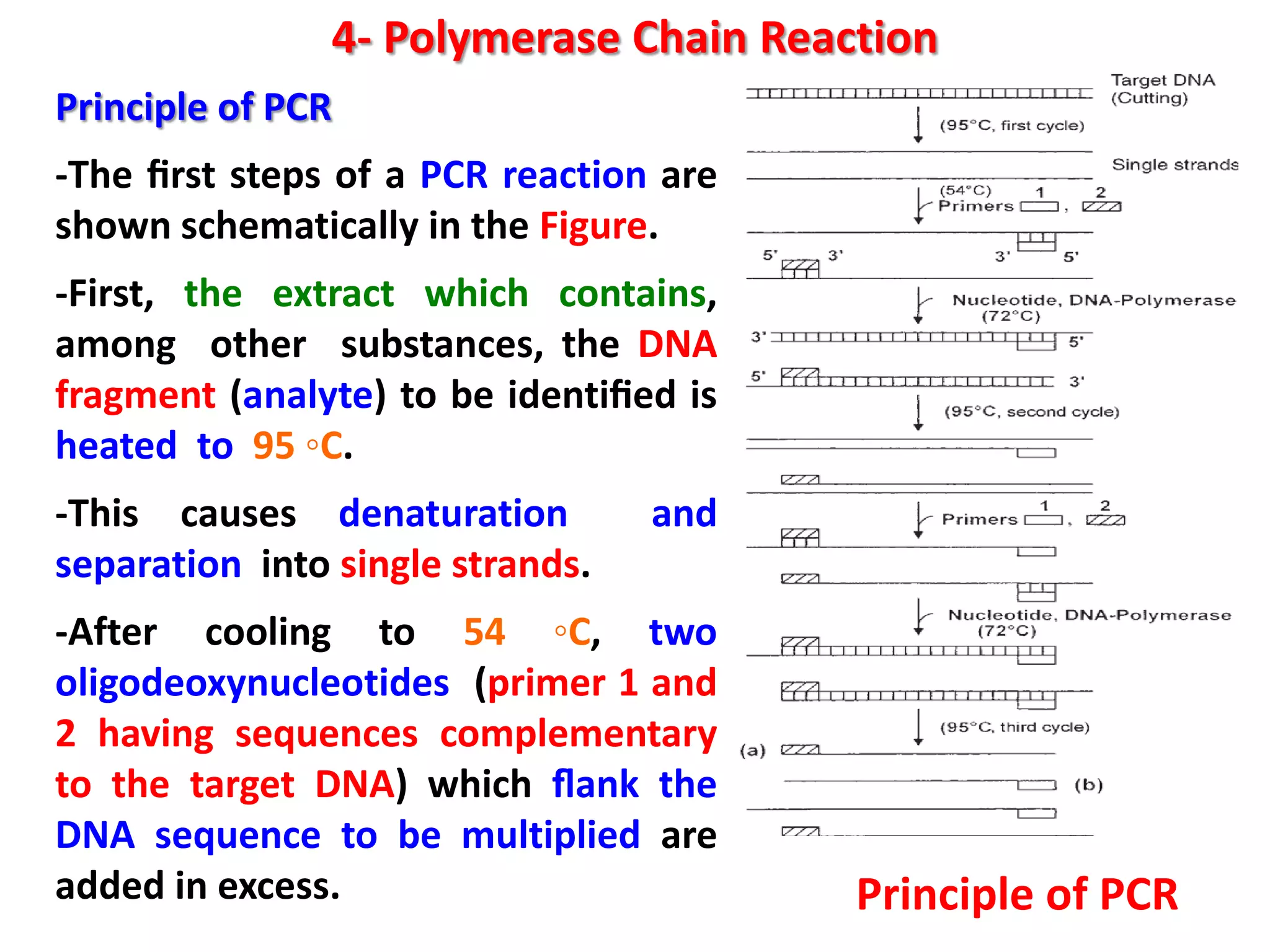
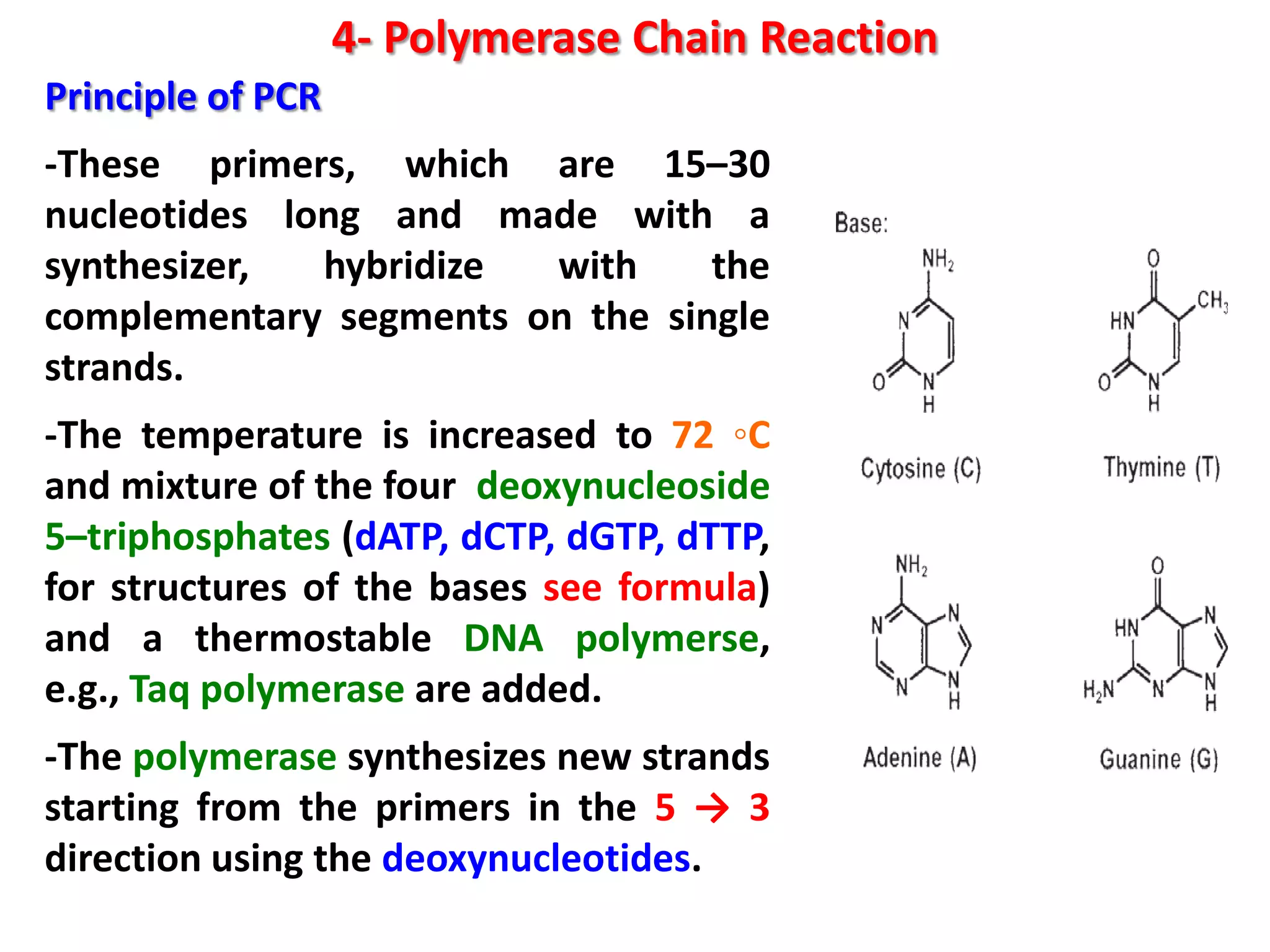
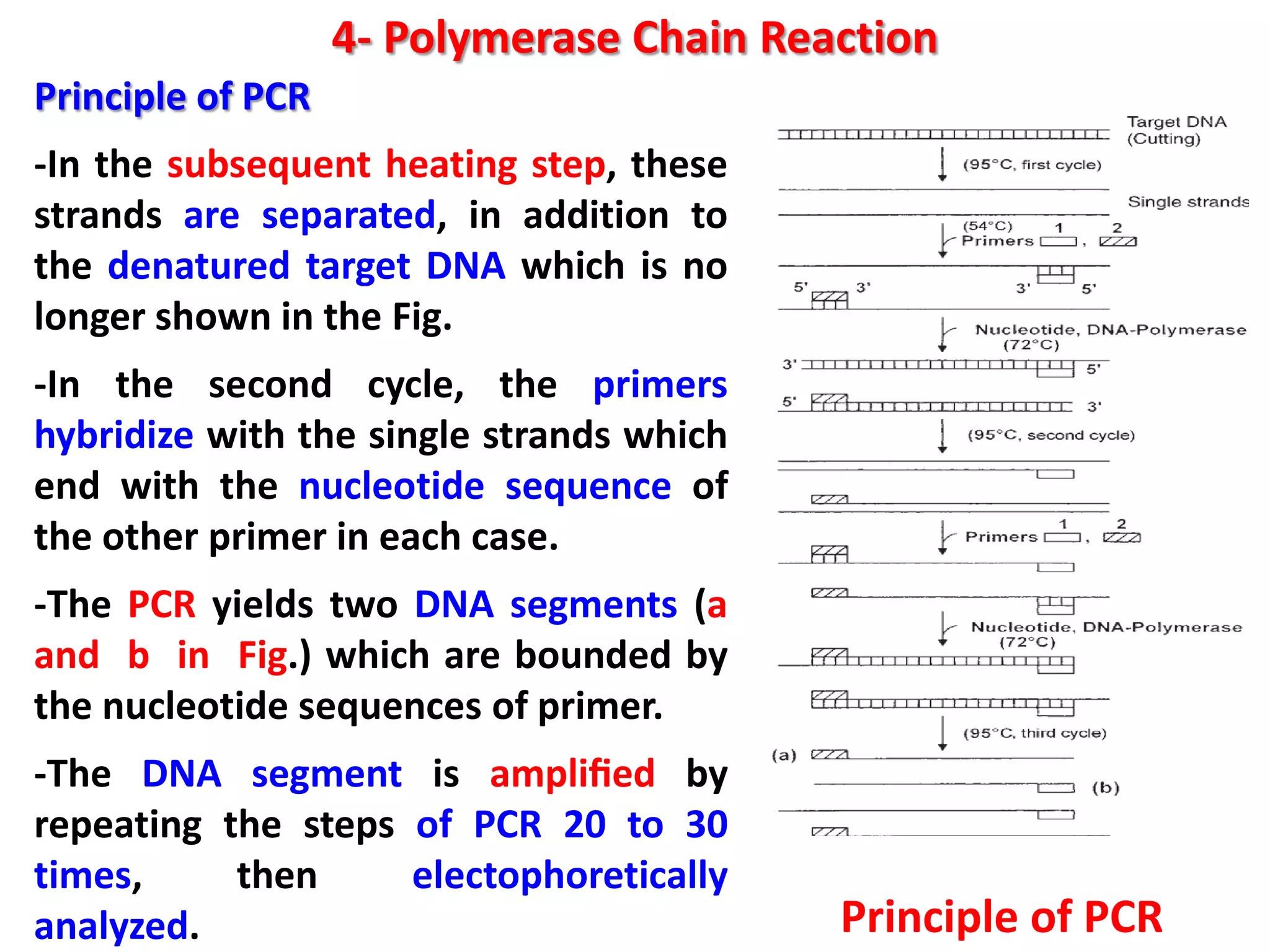
4) Polymerase chain reaction (PCR) which amplifies DNA for sensitive species identification and detecting genetically modified foods. PCR allows analysis of heat-treated foods where proteins