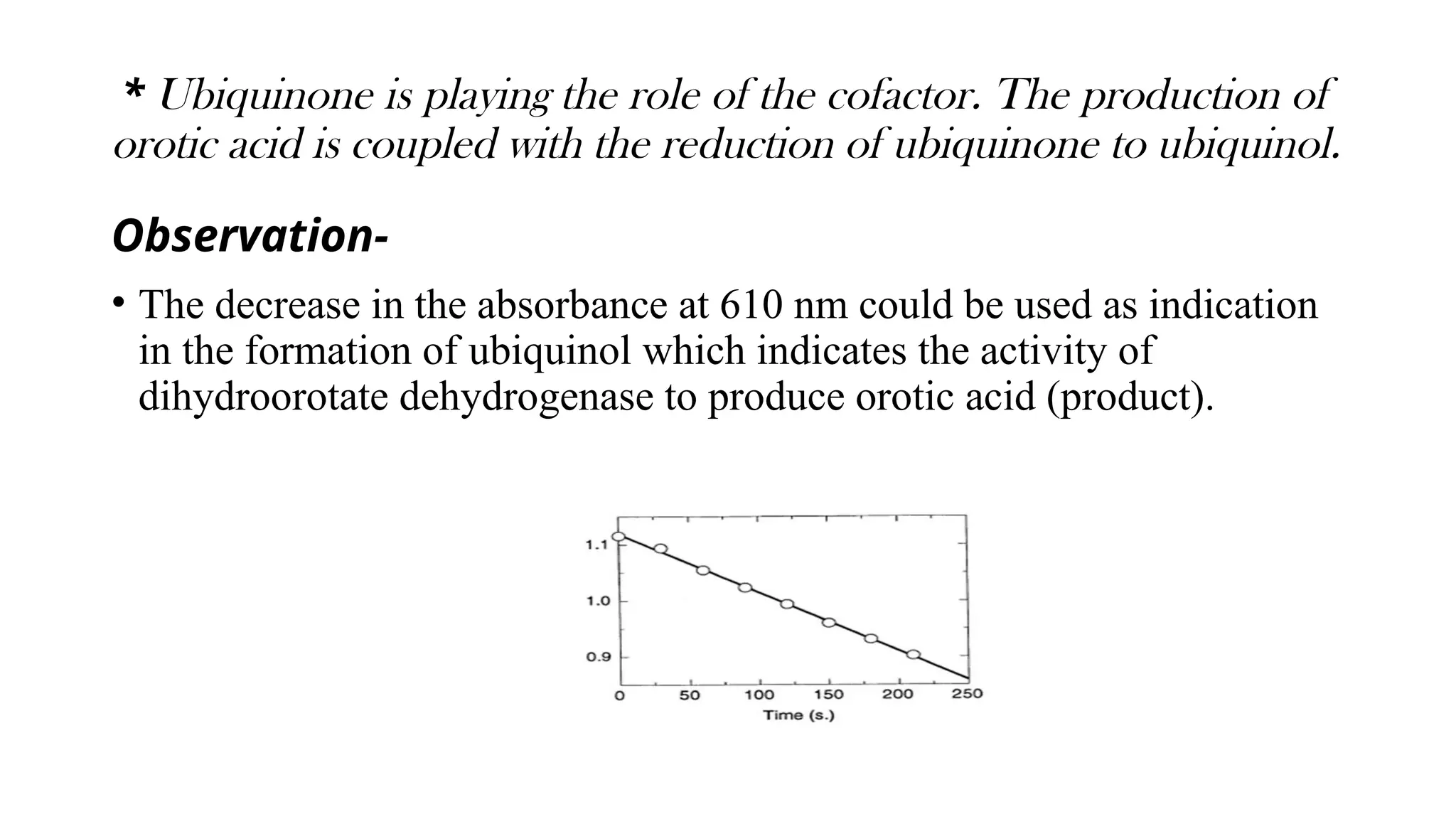
The document discusses various assay strategies for monitoring enzyme activity, focusing on measuring substrate consumption or product formation. It outlines continuous assays (direct and indirect), couple assays, and endpoint assays, detailing their methodologies and examples. Additionally, the enzyme alpha-amylase is highlighted, including its activity, characteristics, and applications in carbohydrate hydrolysis.