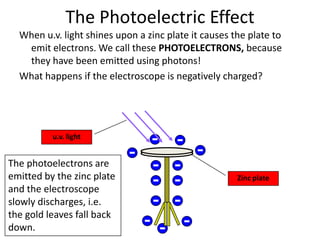
- The document discusses the photoelectric effect where ultraviolet (UV) light causes a zinc plate to emit electrons called photoelectrons.
- It describes several experiments where changing the intensity and frequency of the UV light impacts the number and kinetic energy of the emitted photoelectrons.
- Key concepts explained include the work function, which is the minimum energy needed to remove an electron from a metal, and how it differs for different metals. The threshold frequency is the minimum frequency needed to cause photoemission for a given metal.