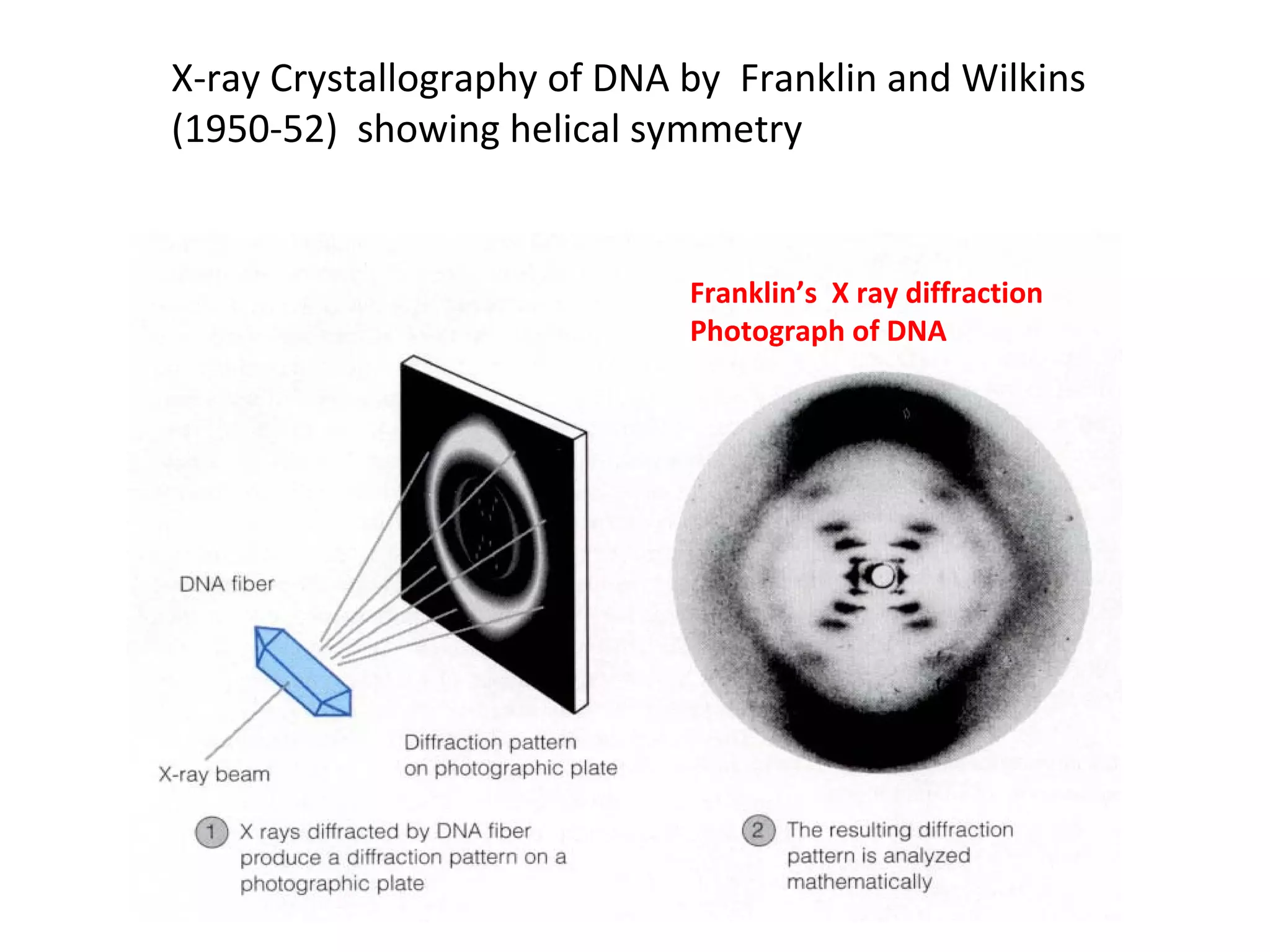
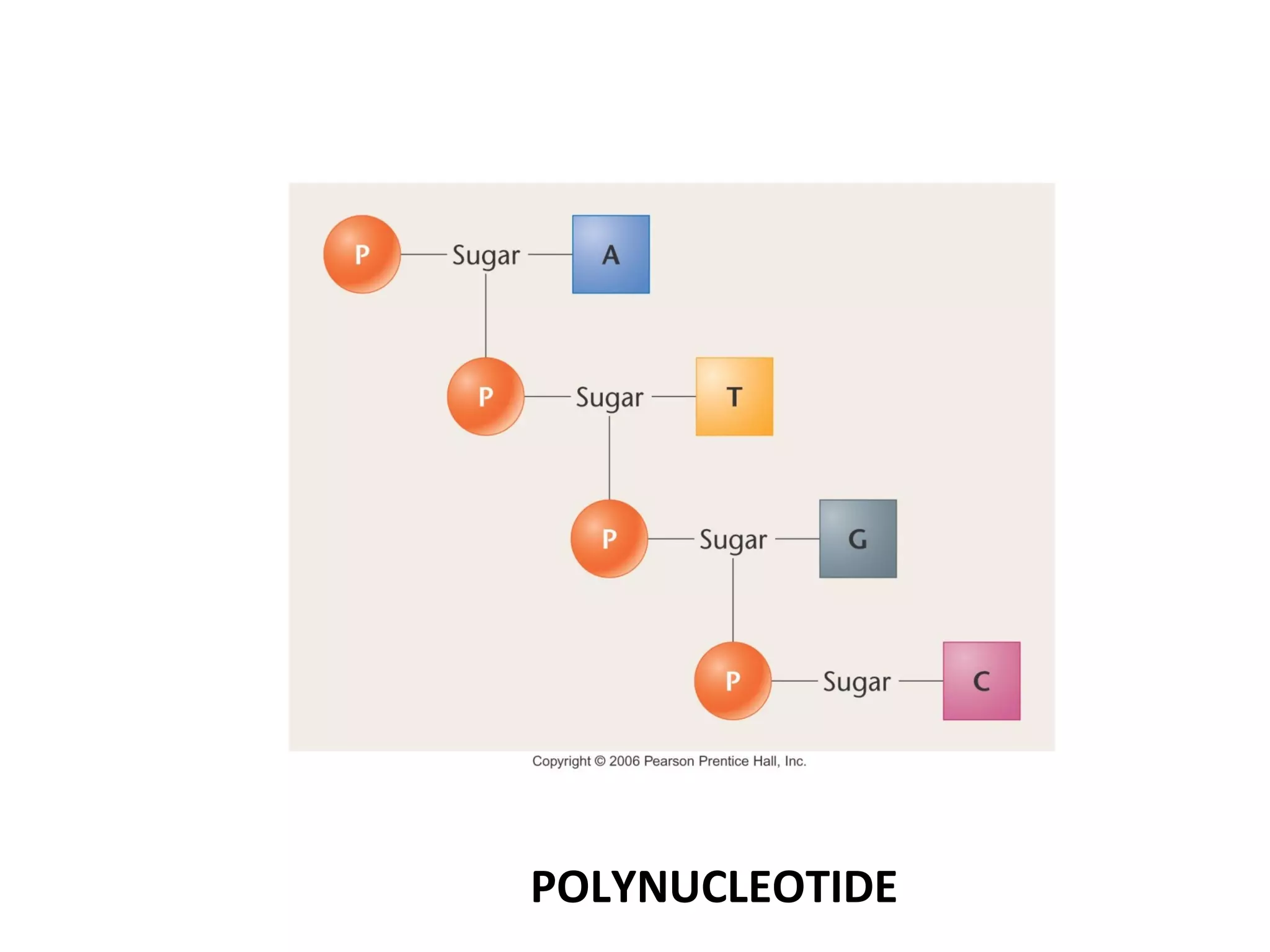
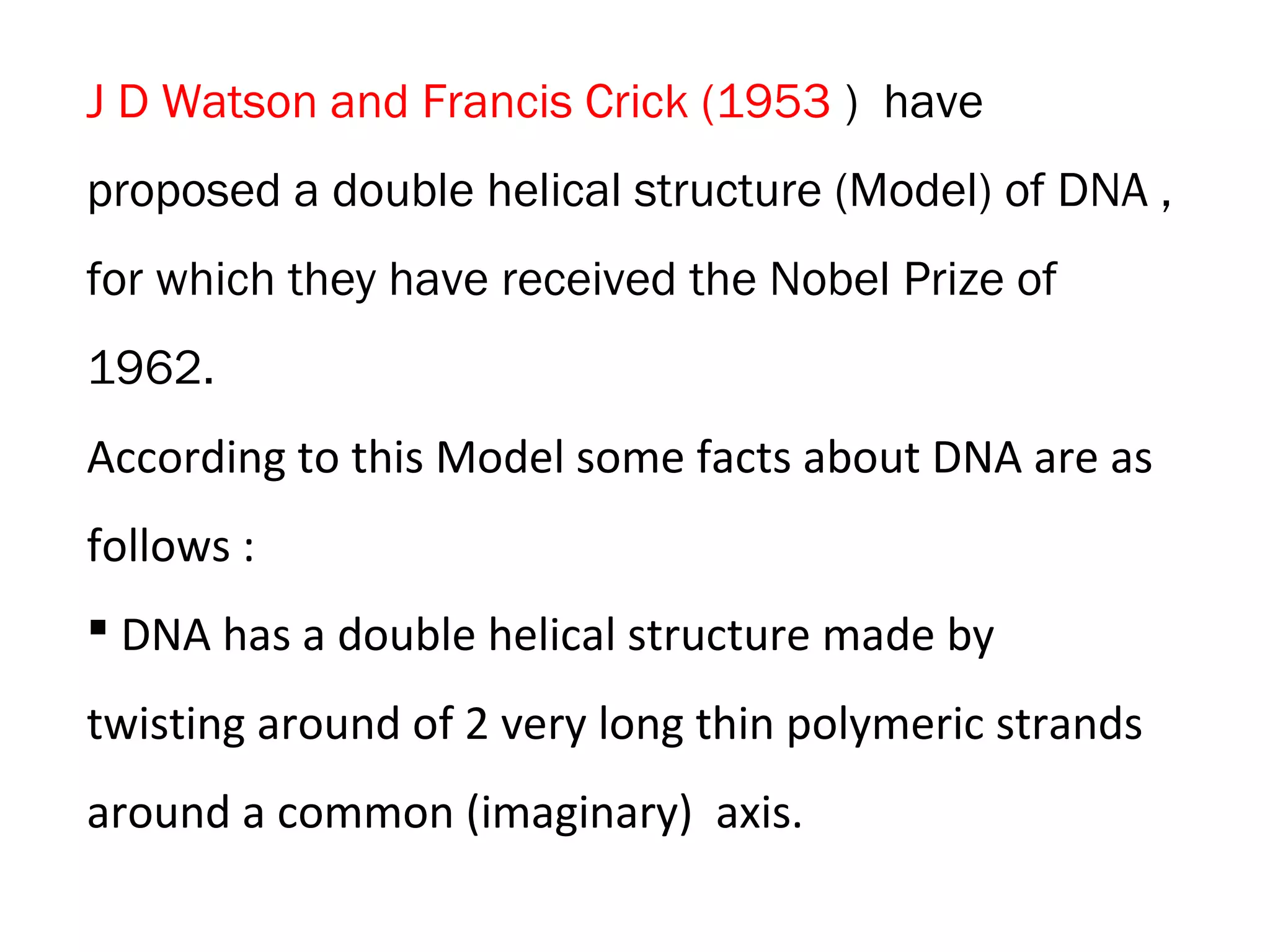
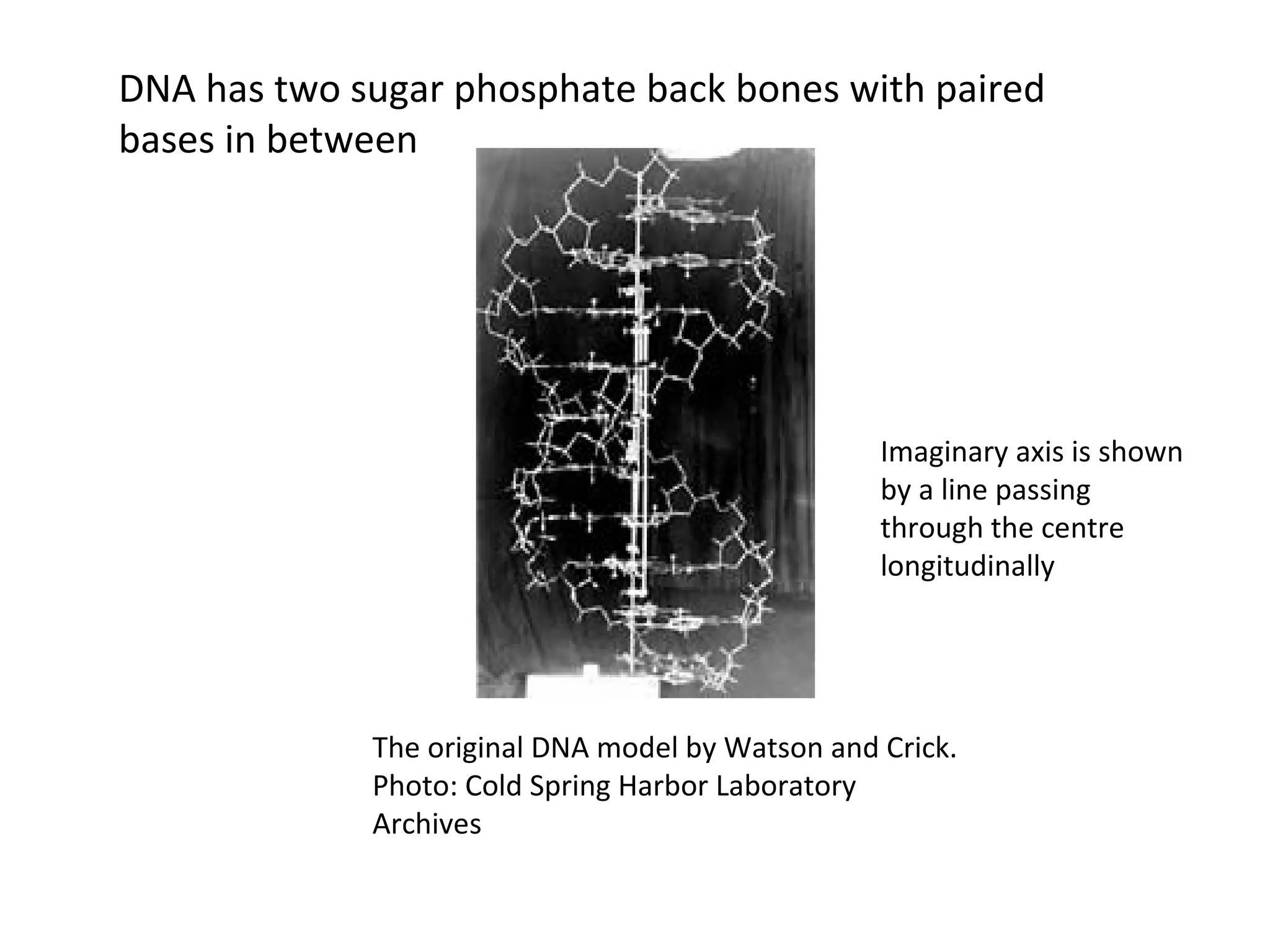
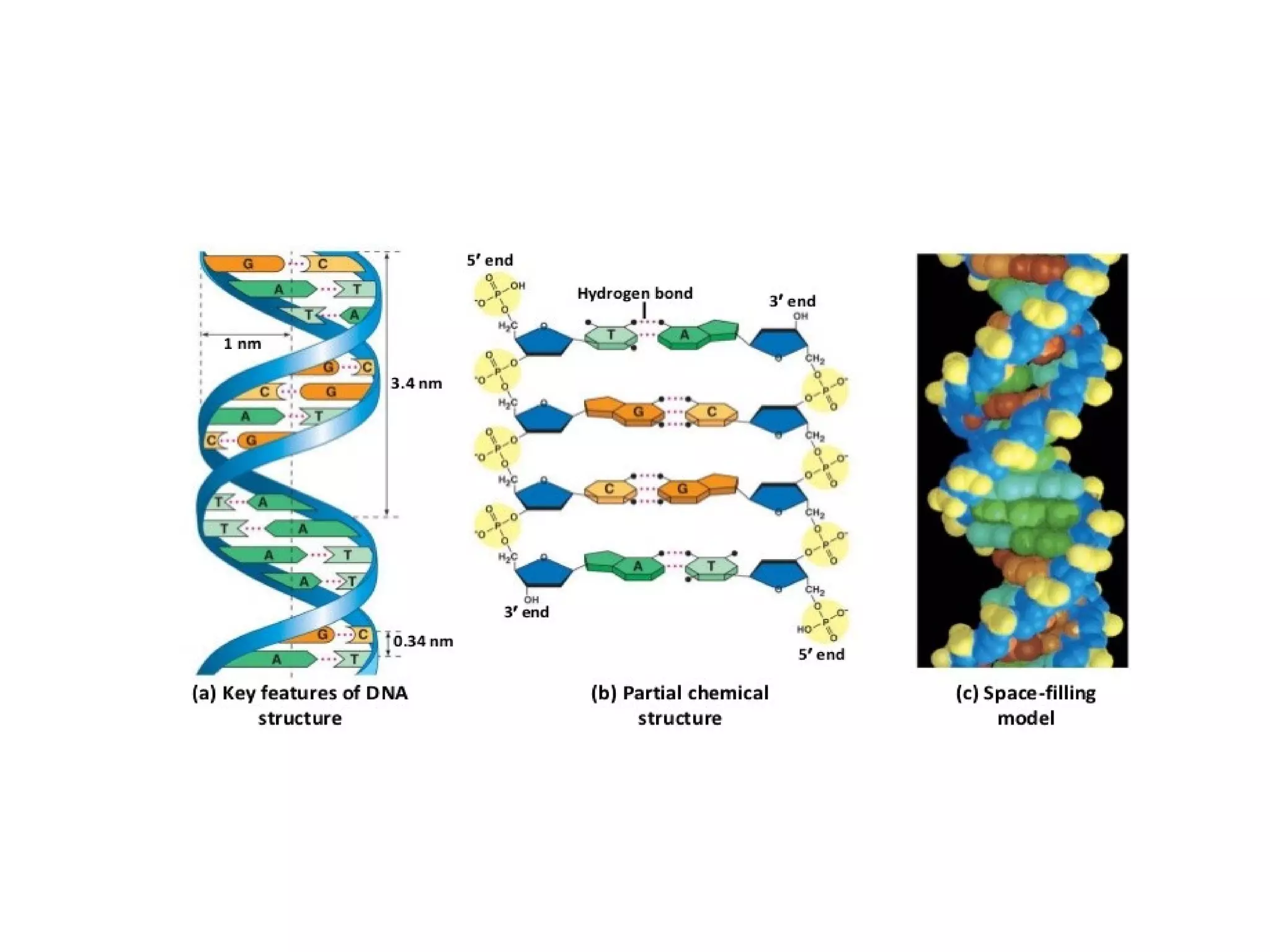
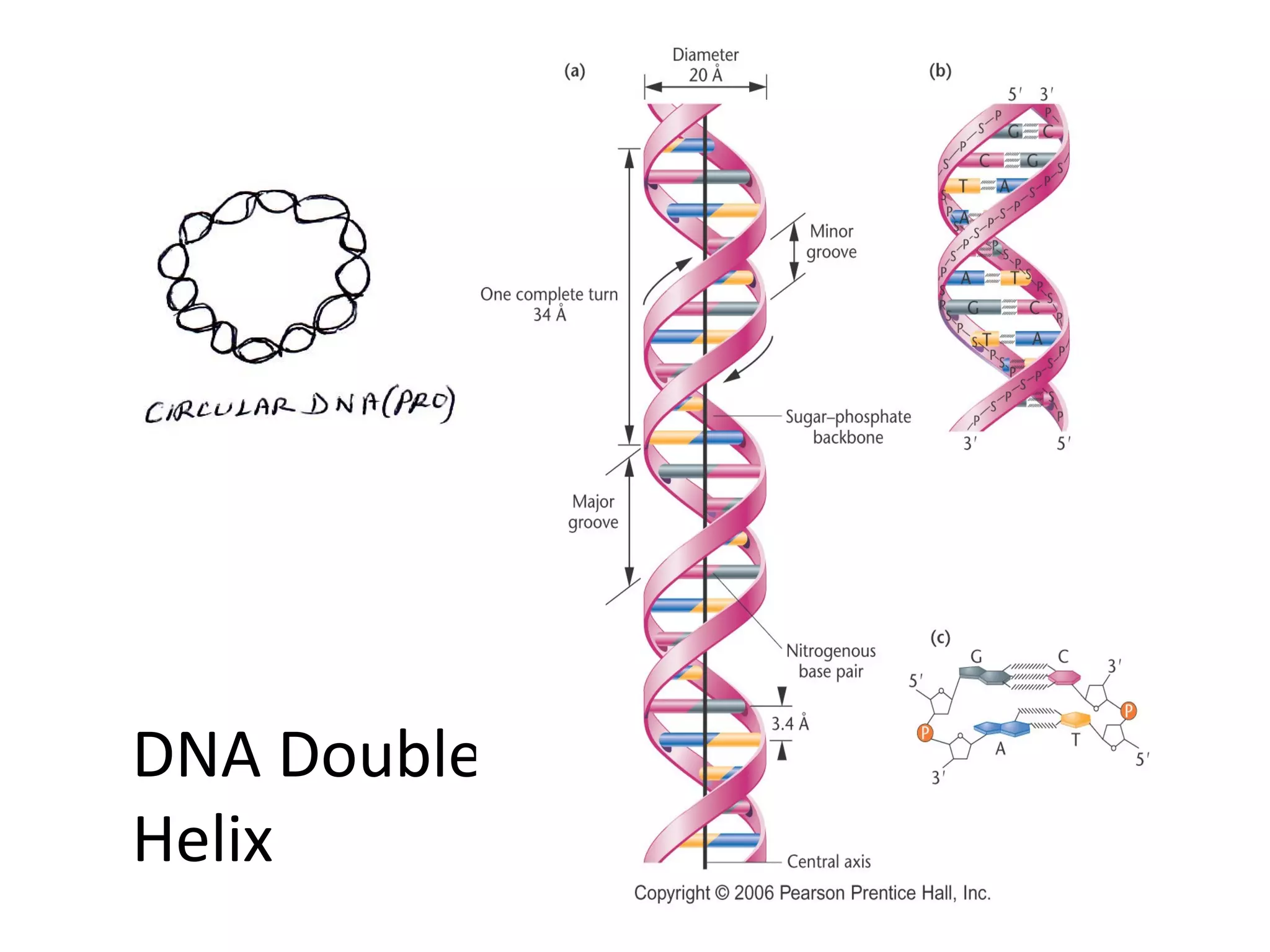
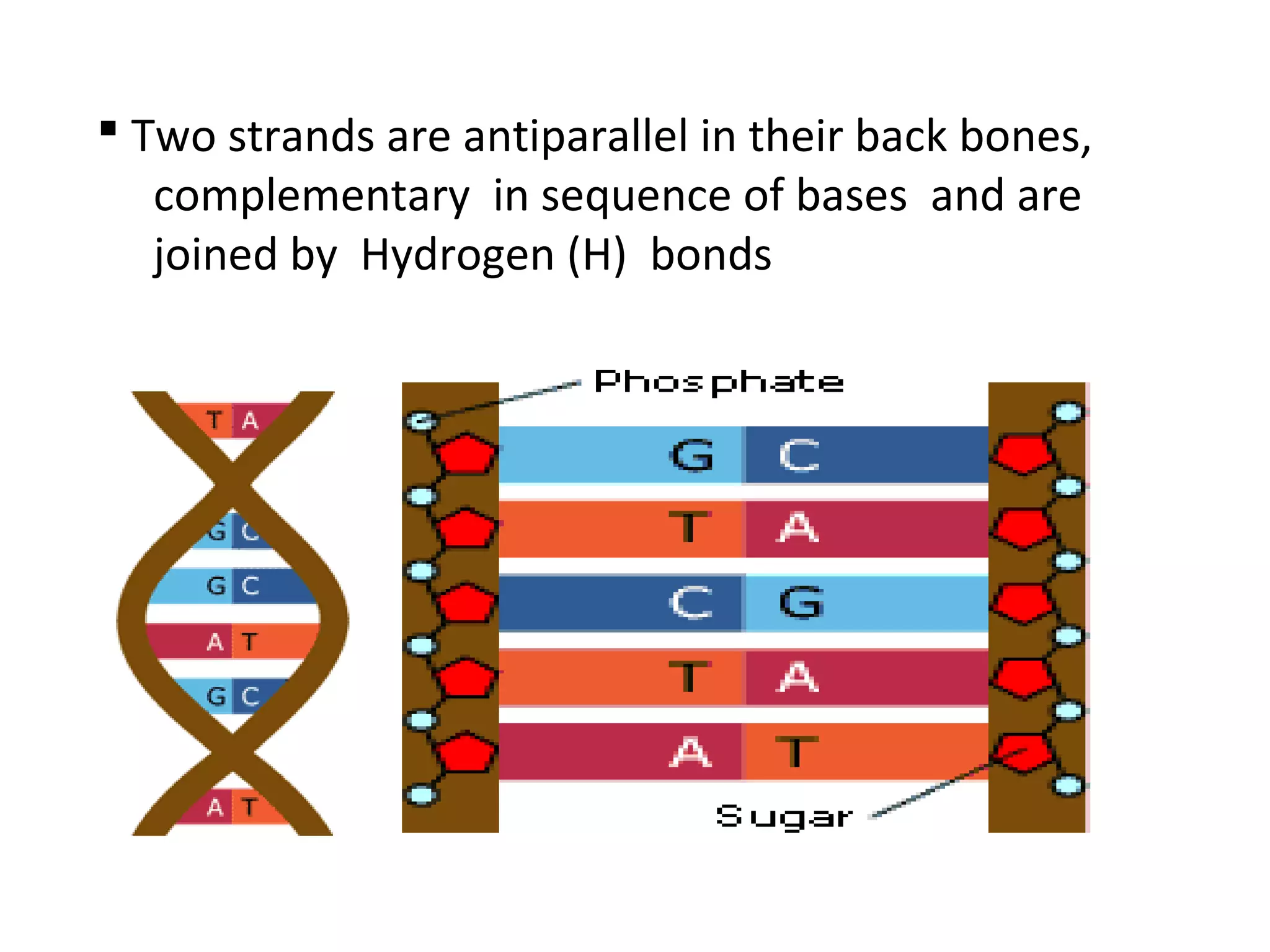
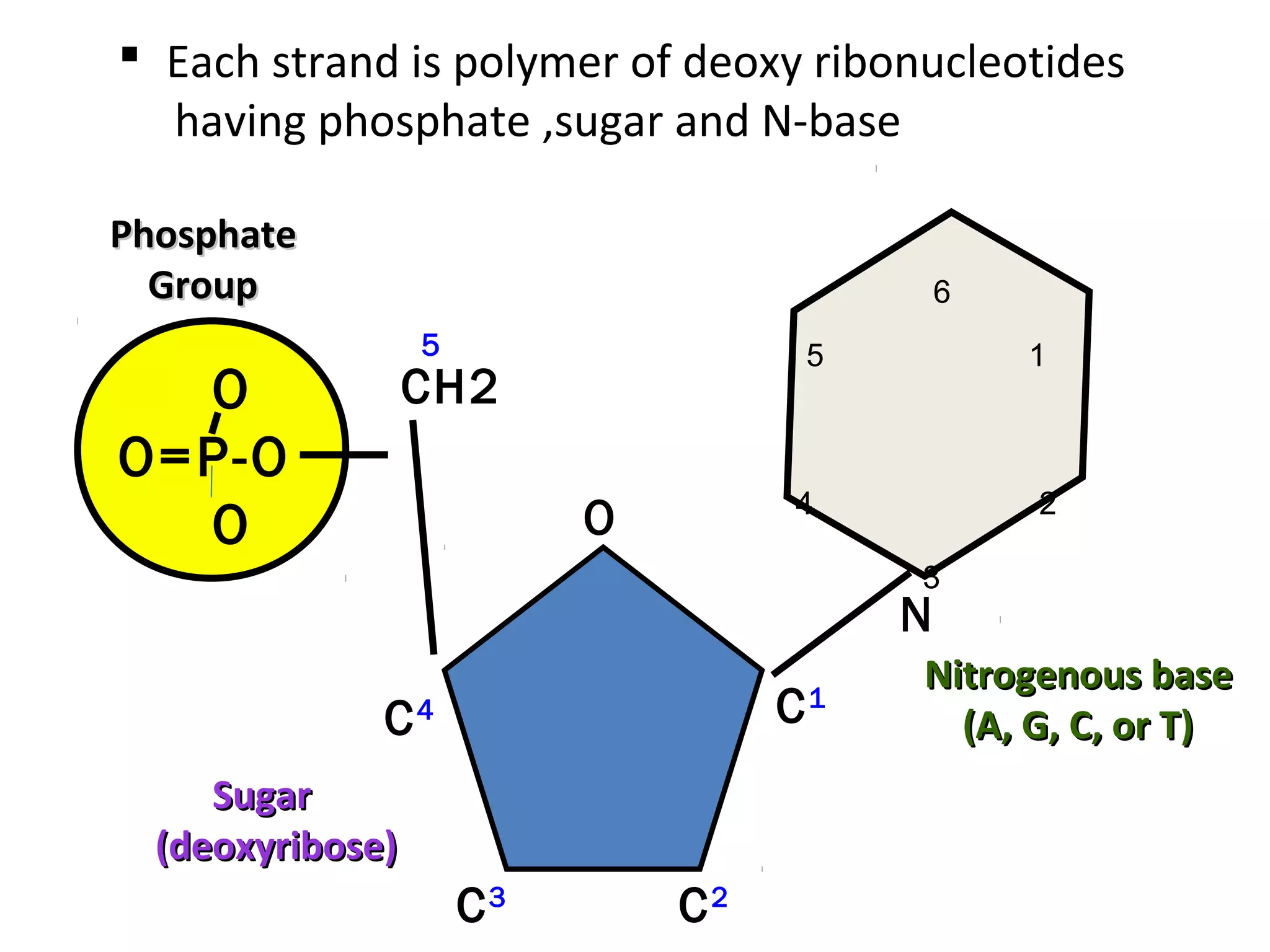
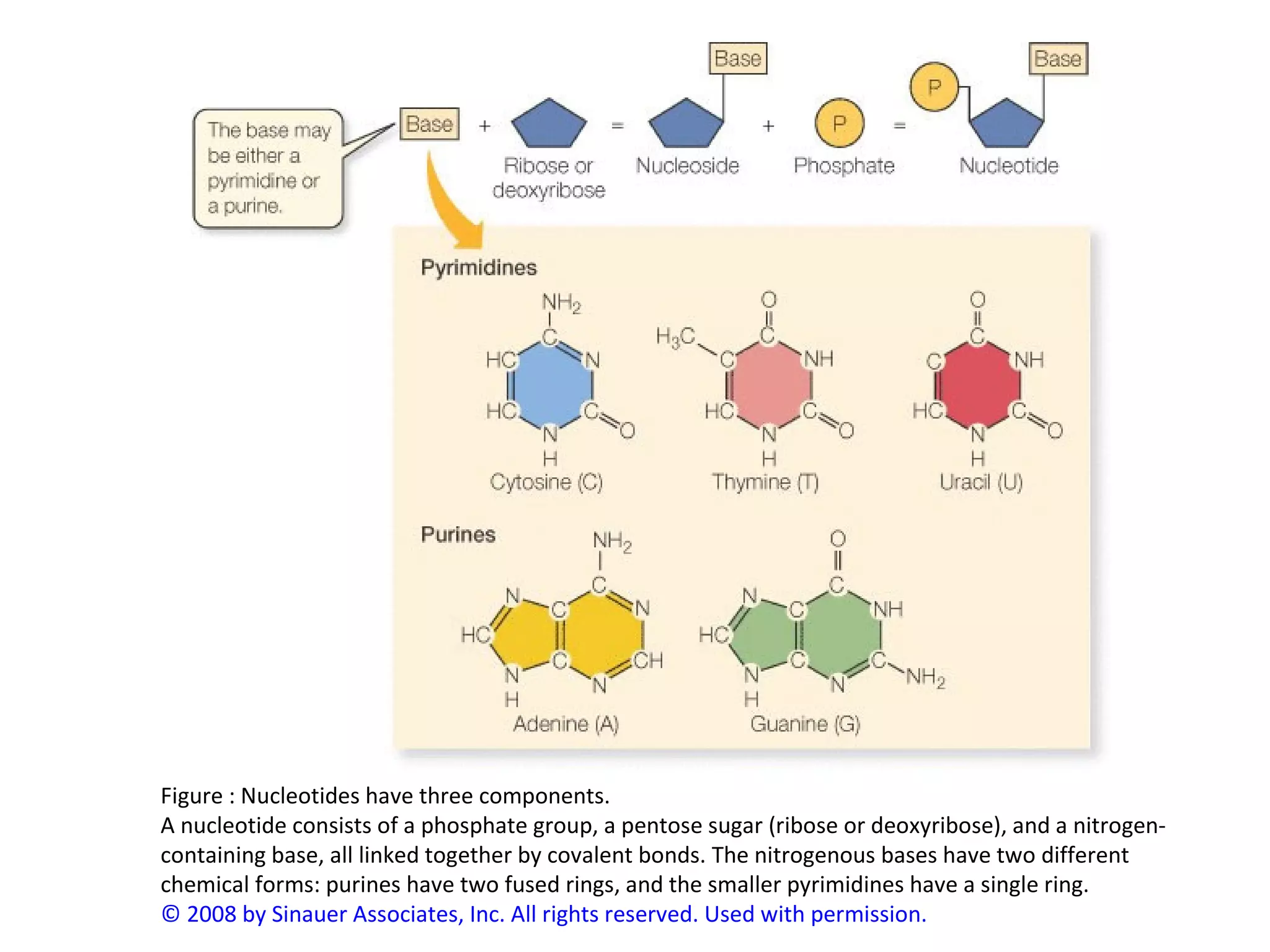
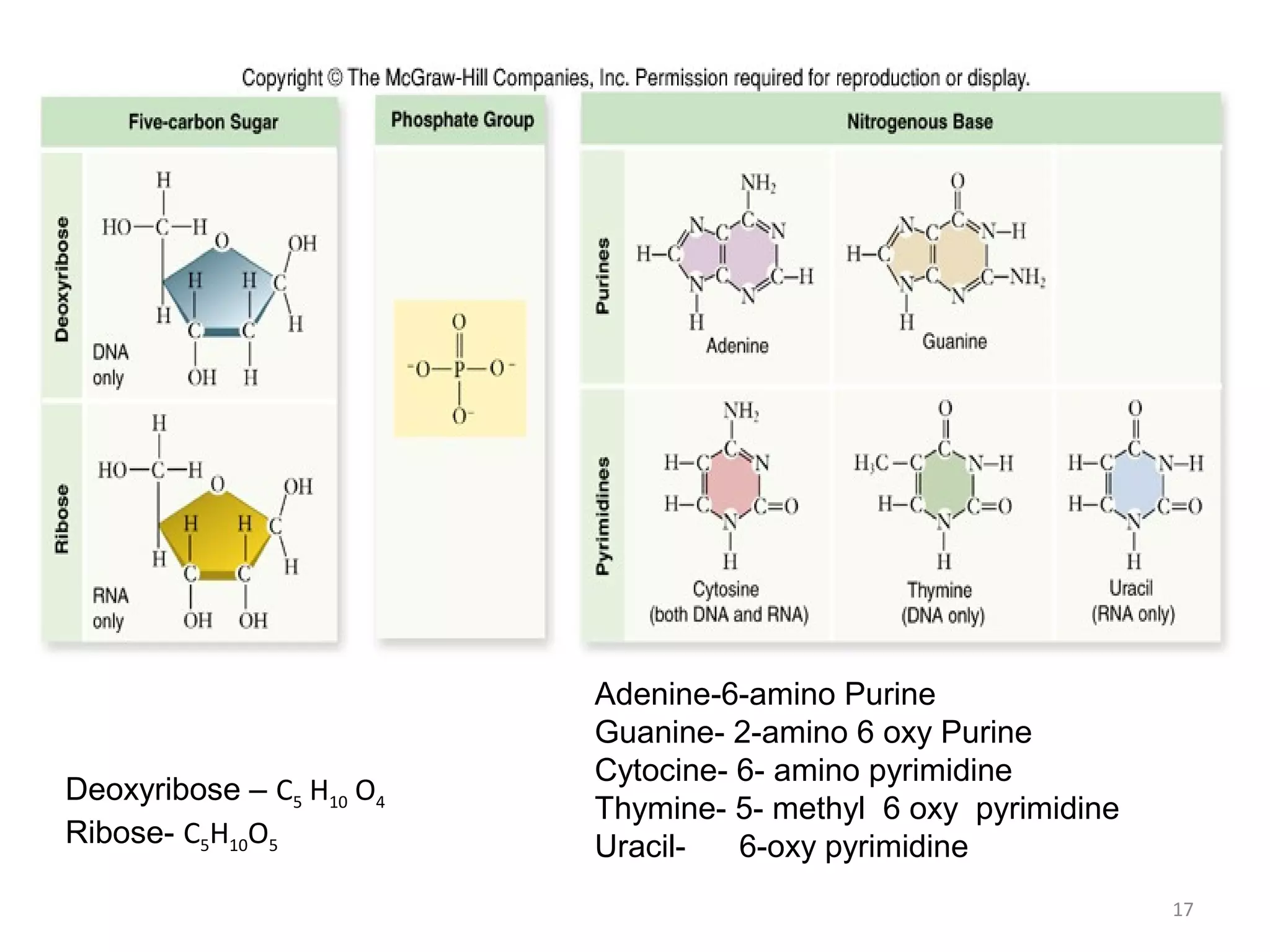
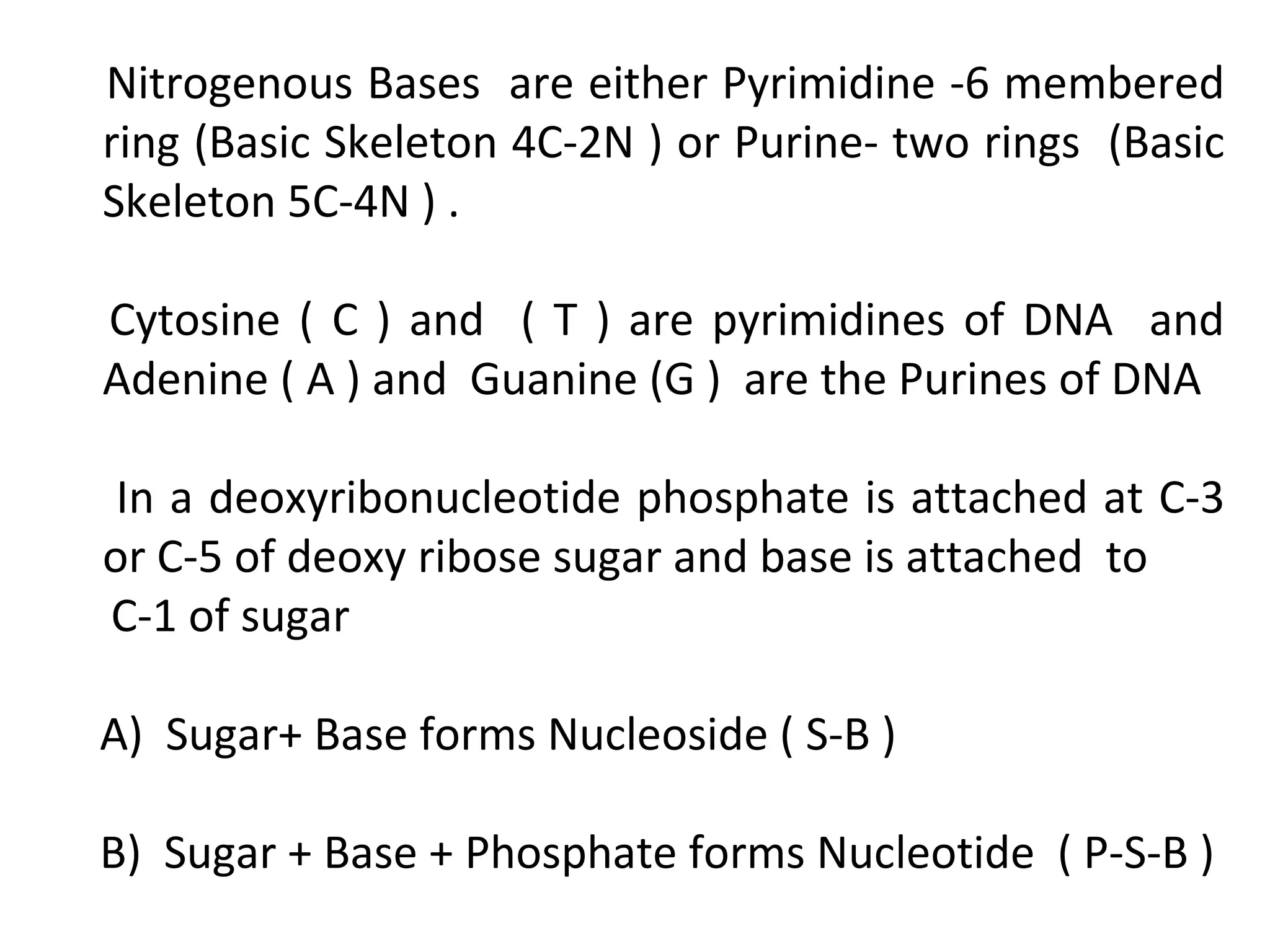
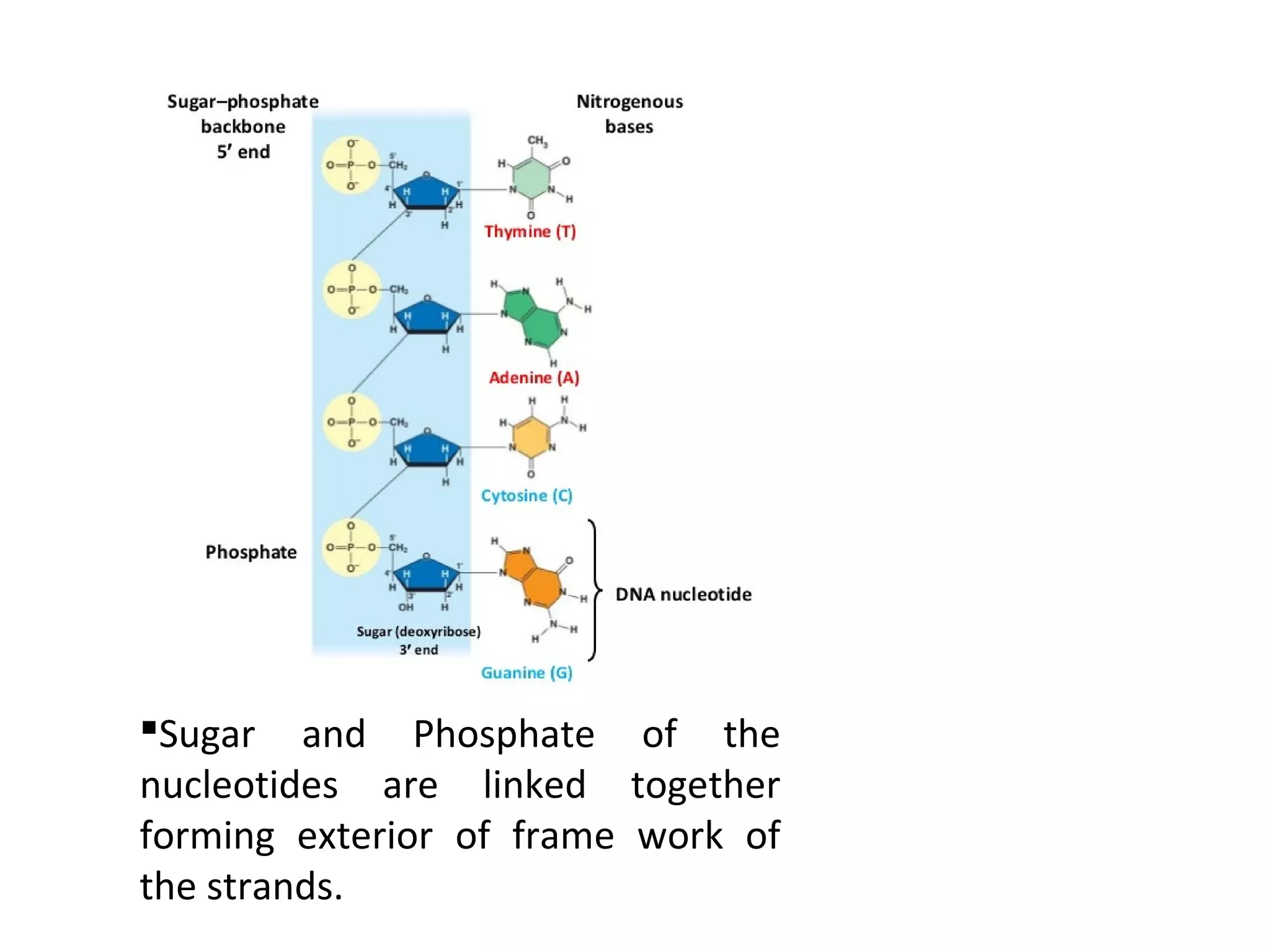
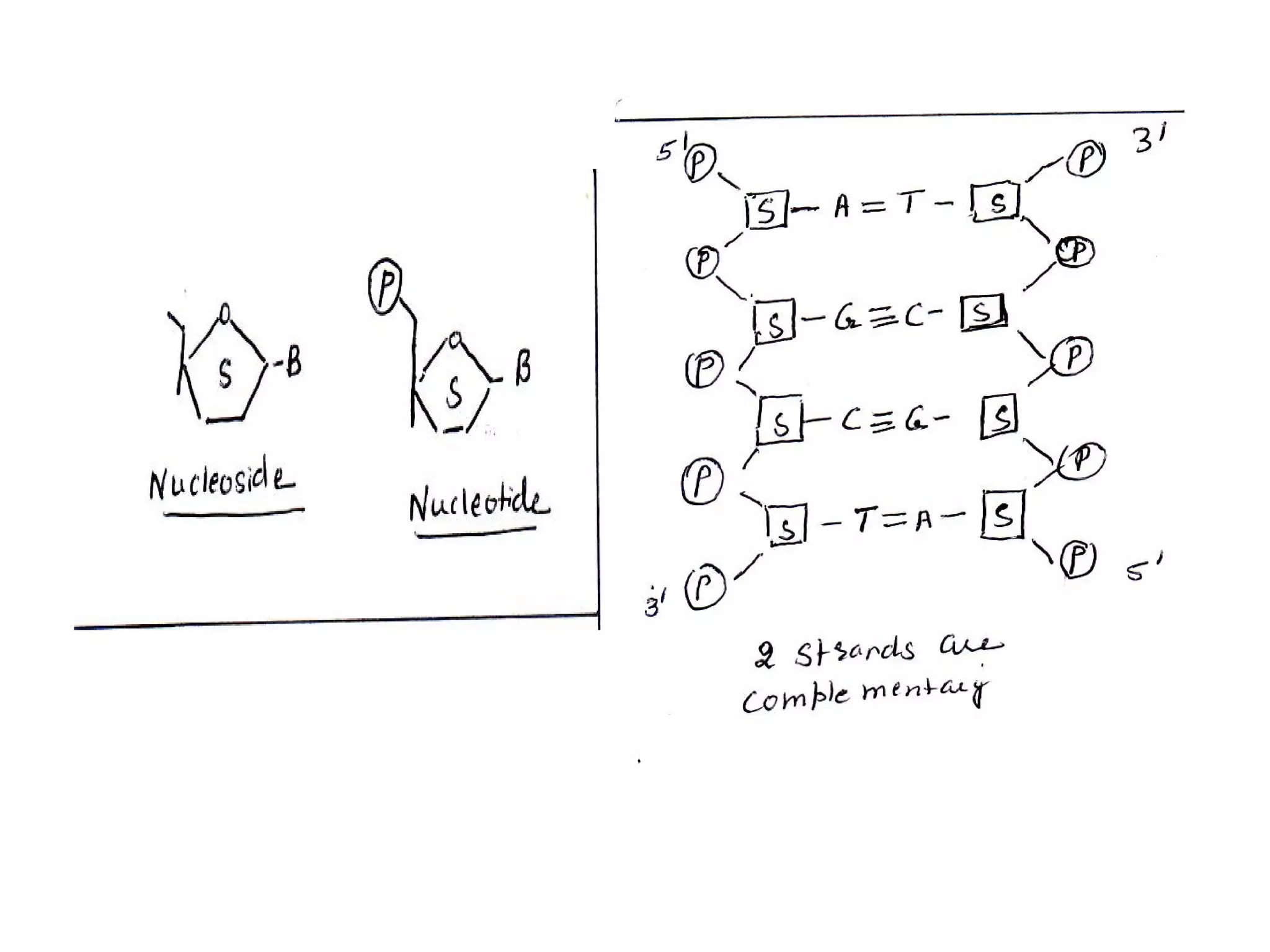
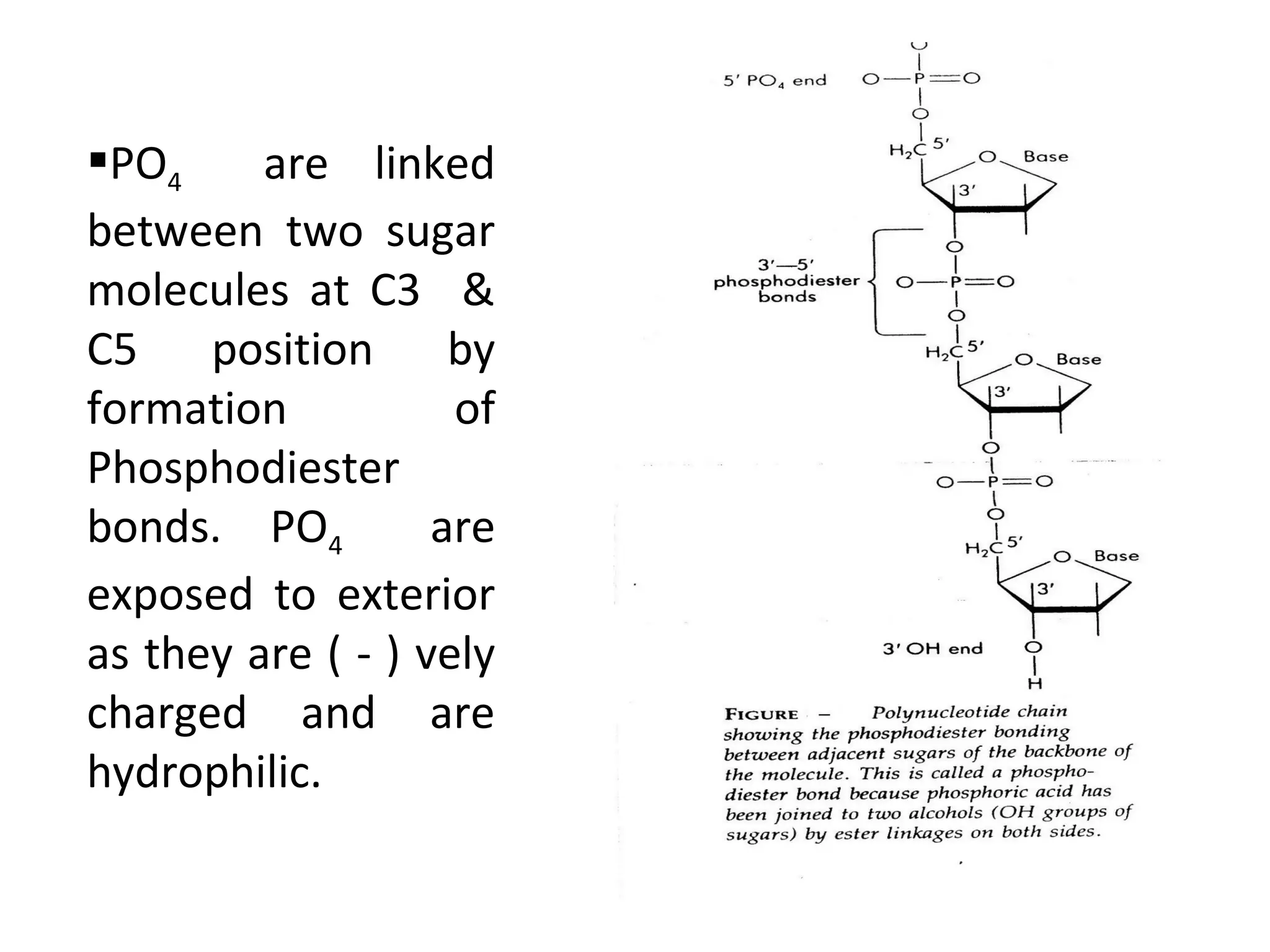
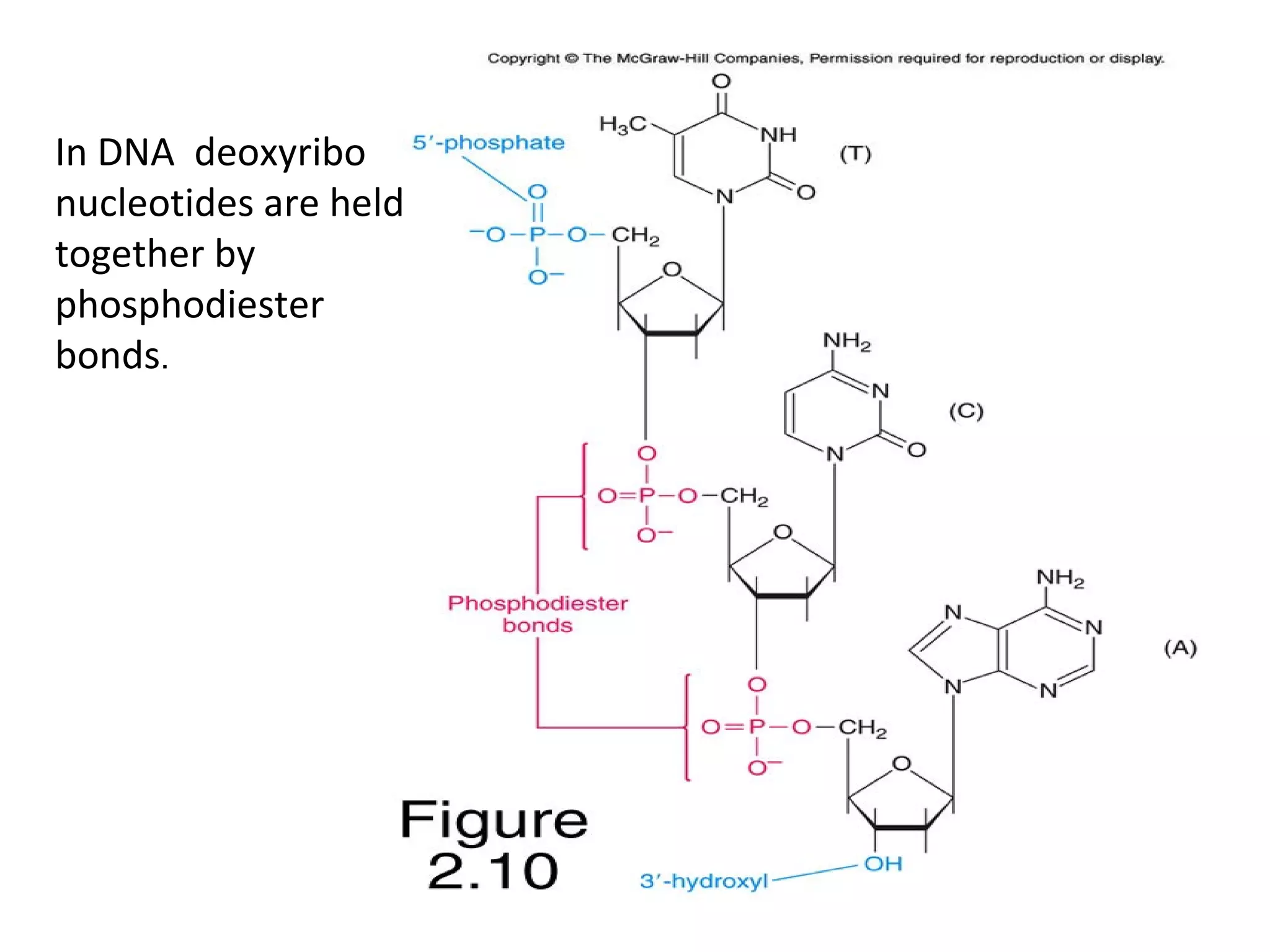
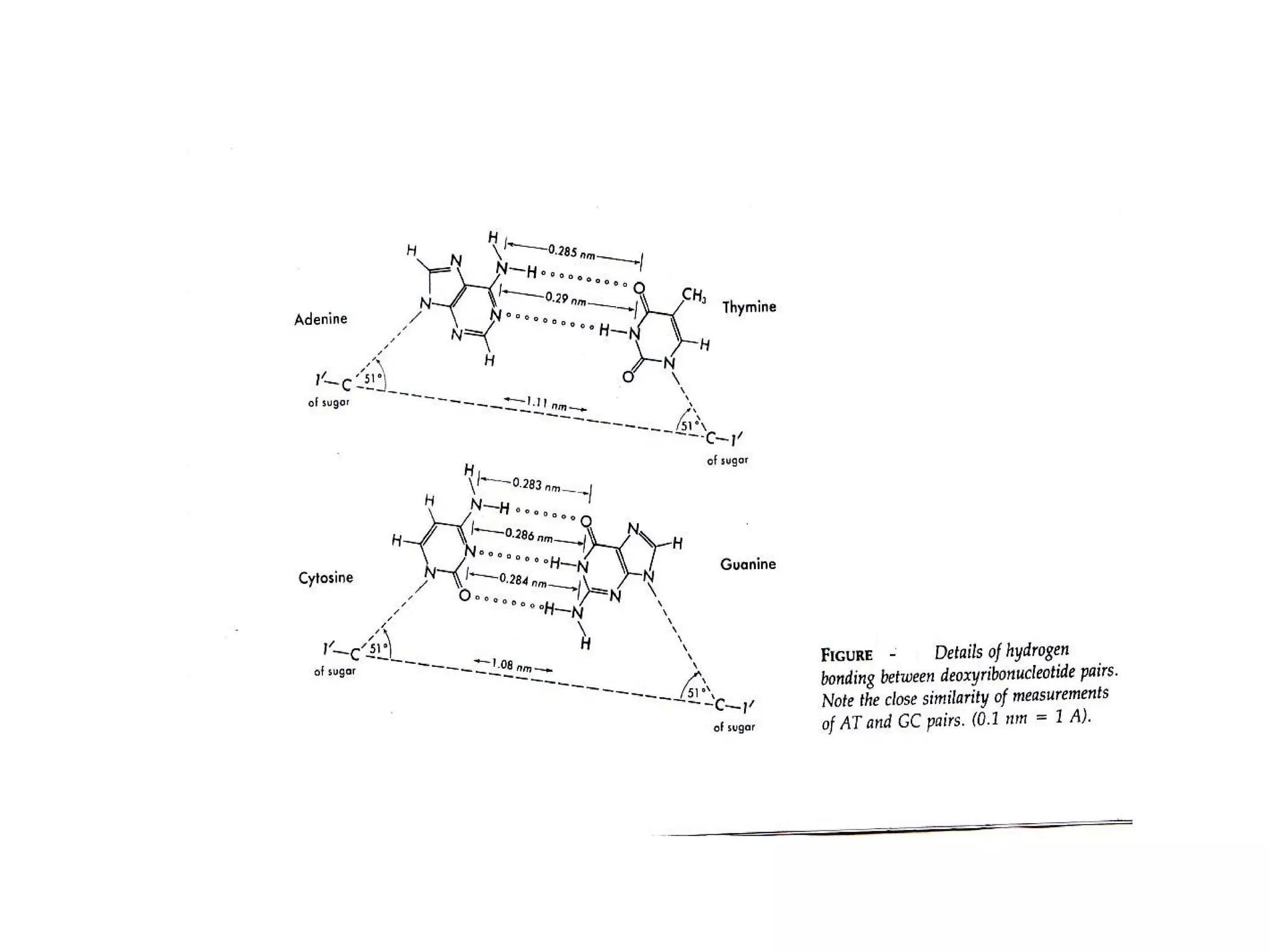
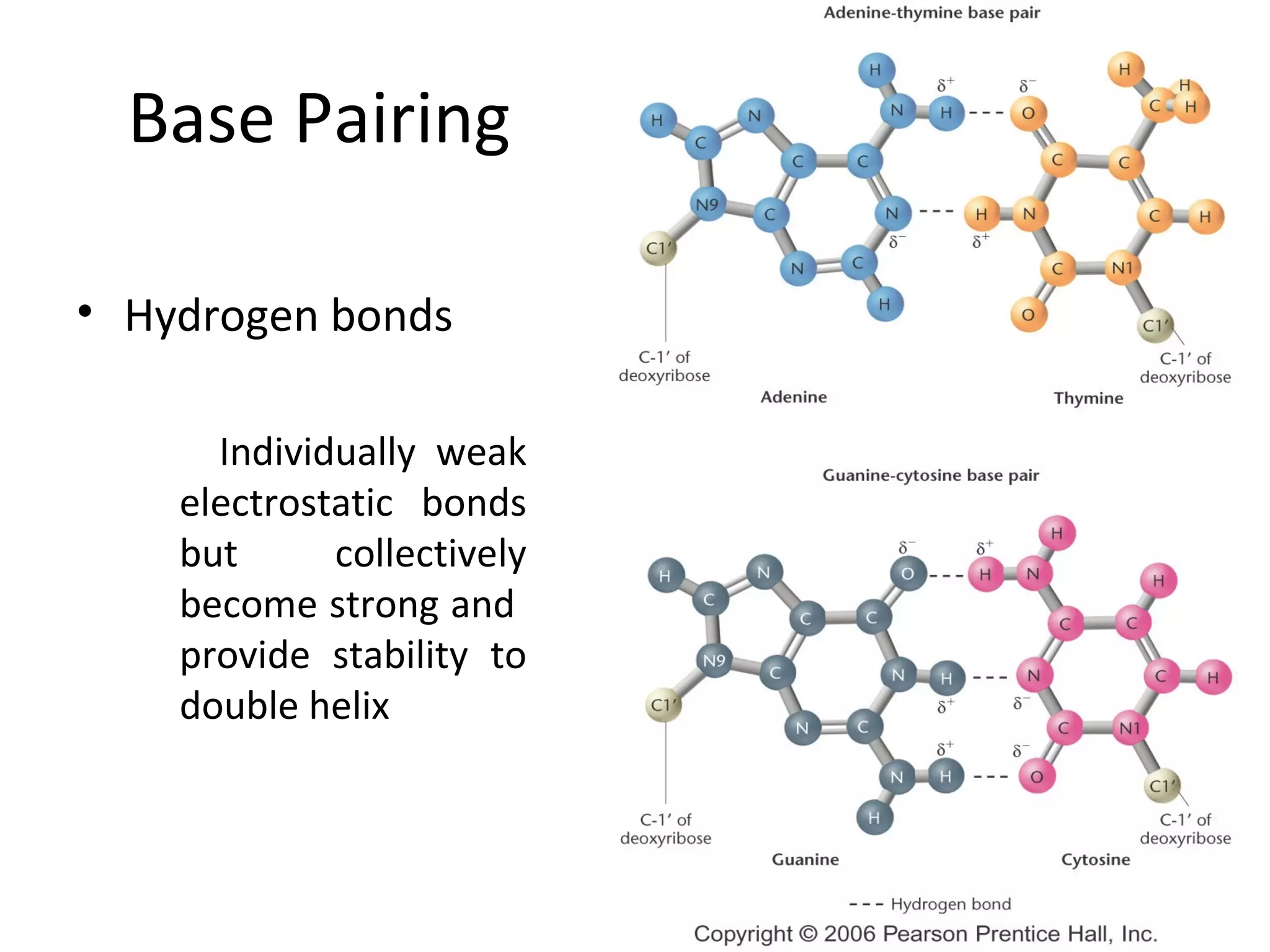
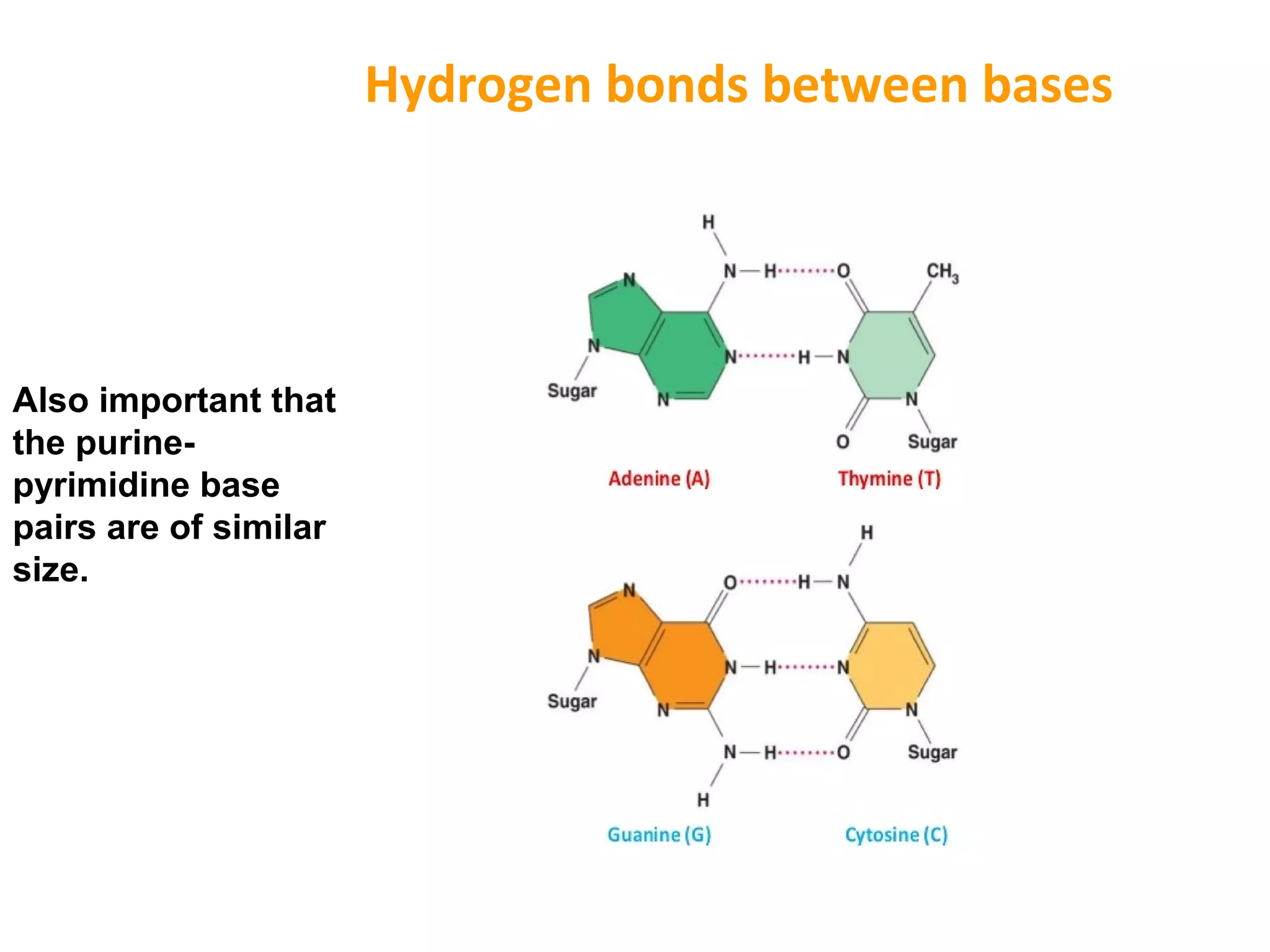
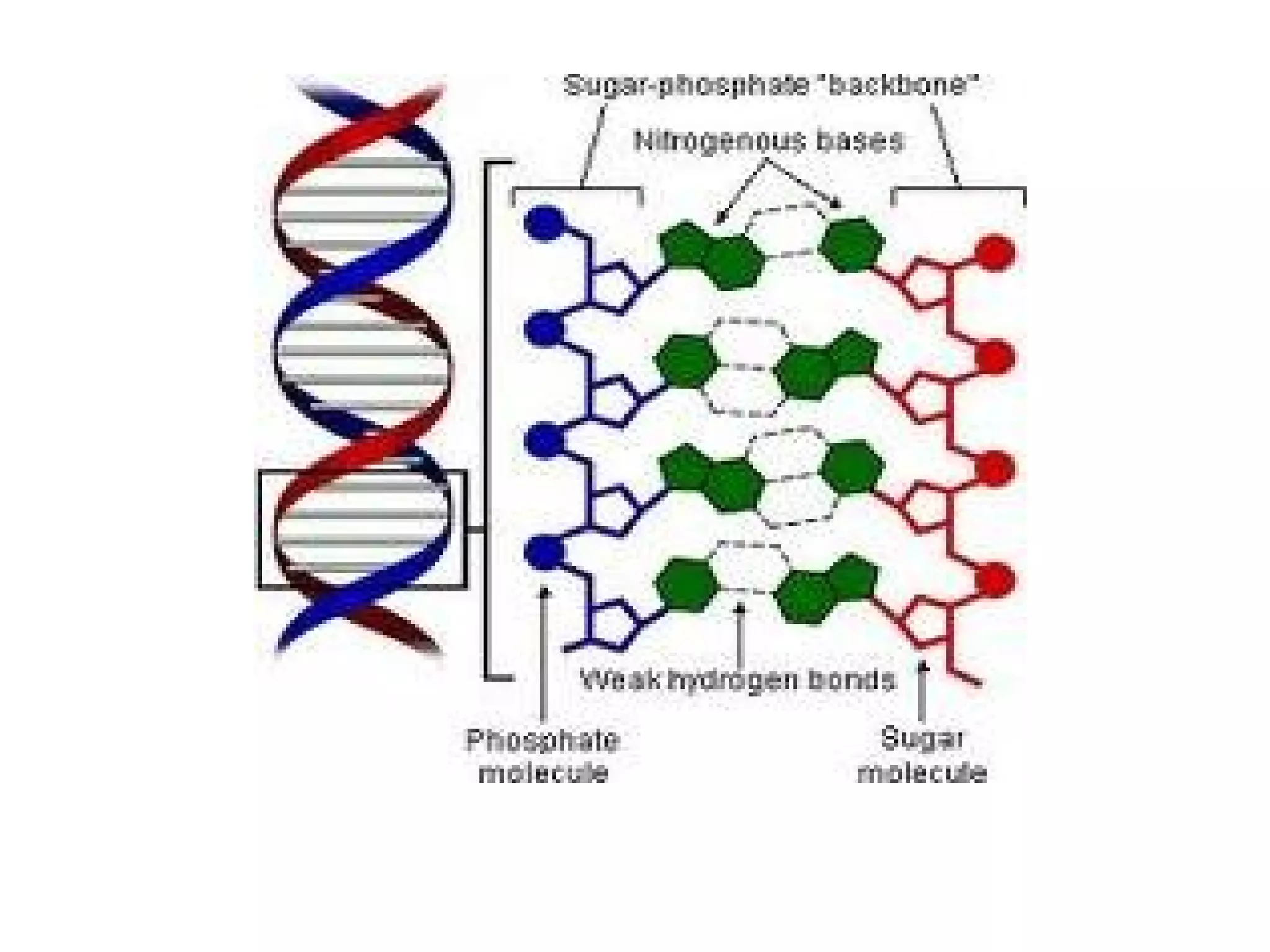
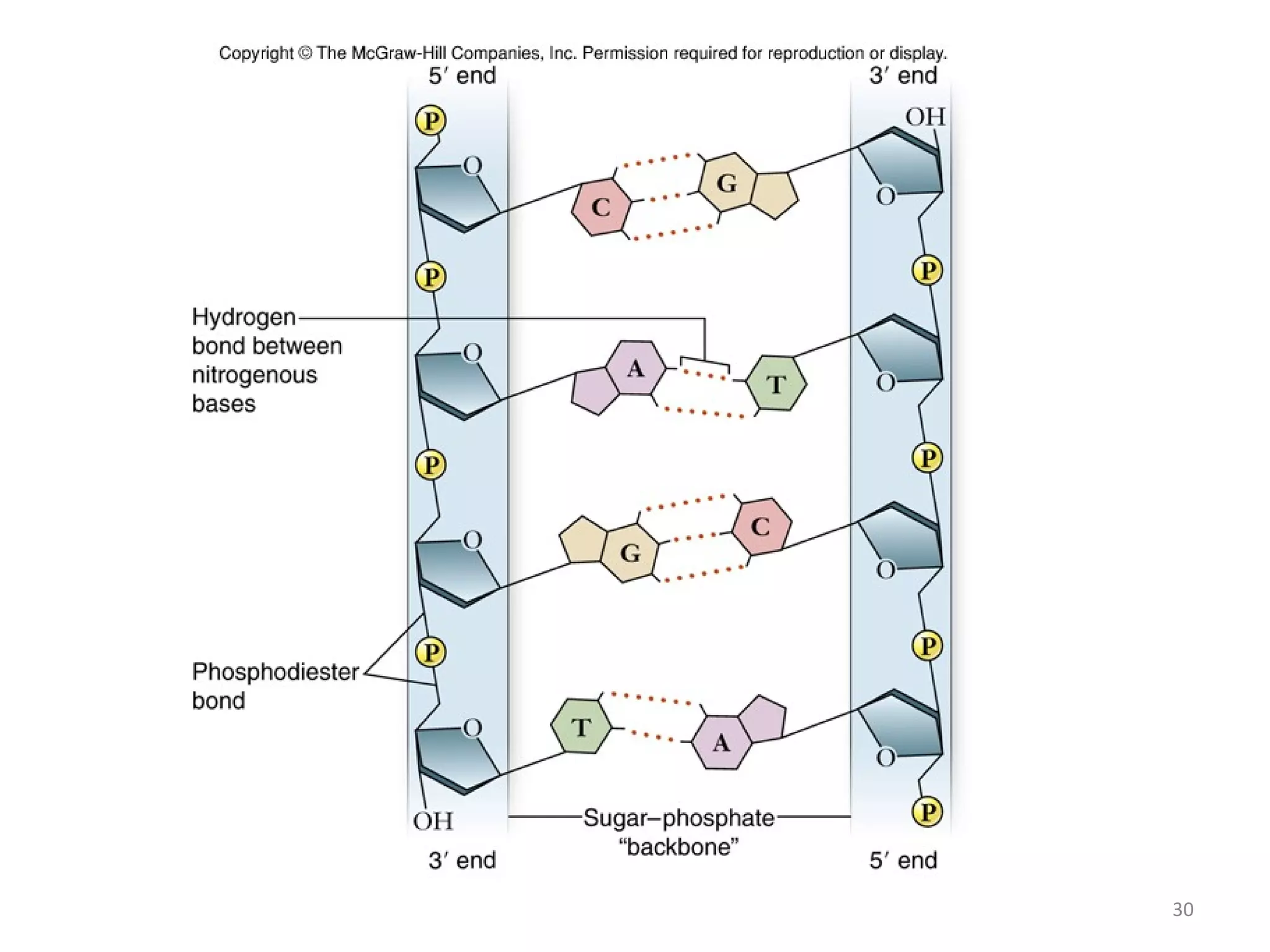
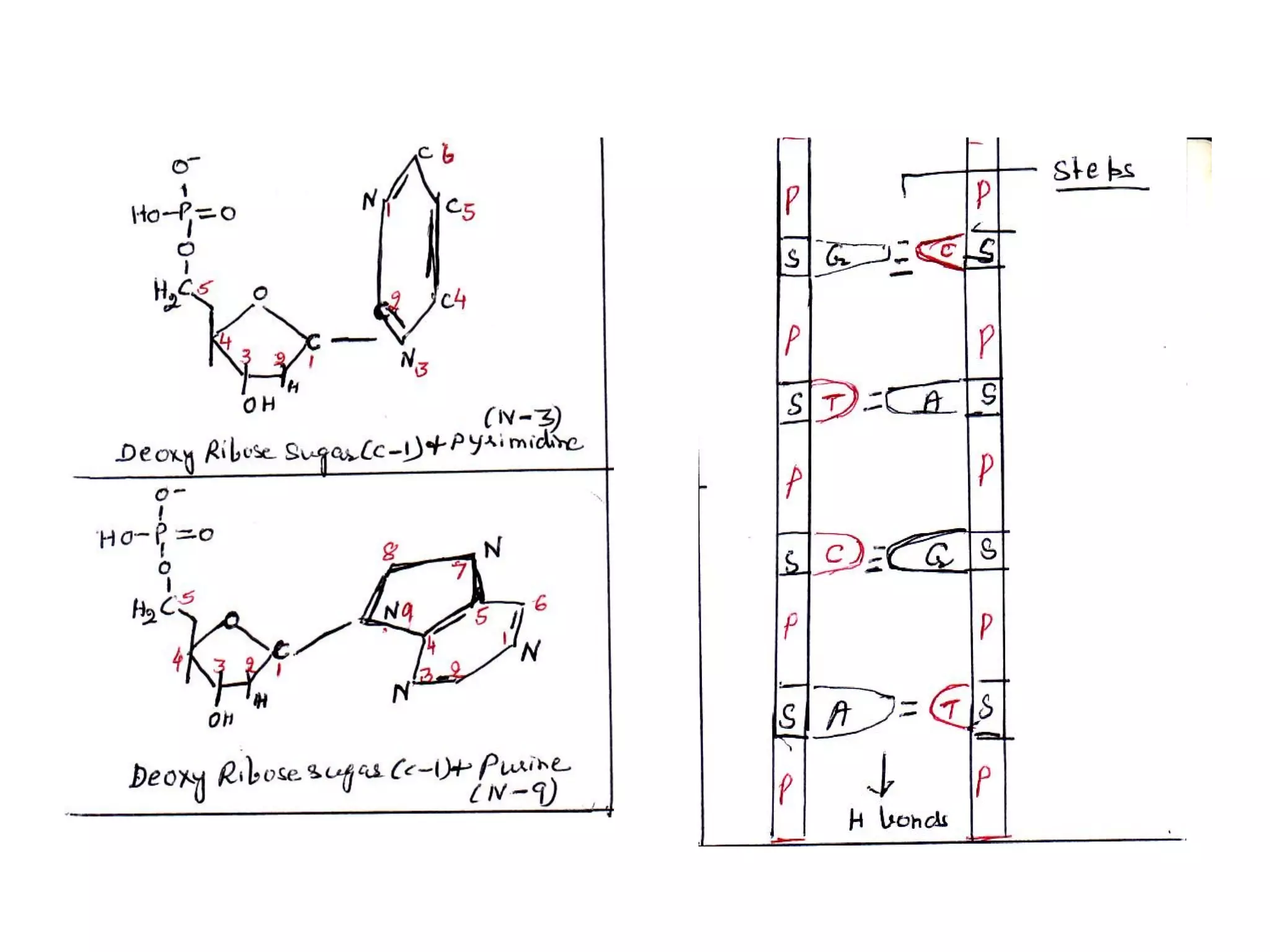
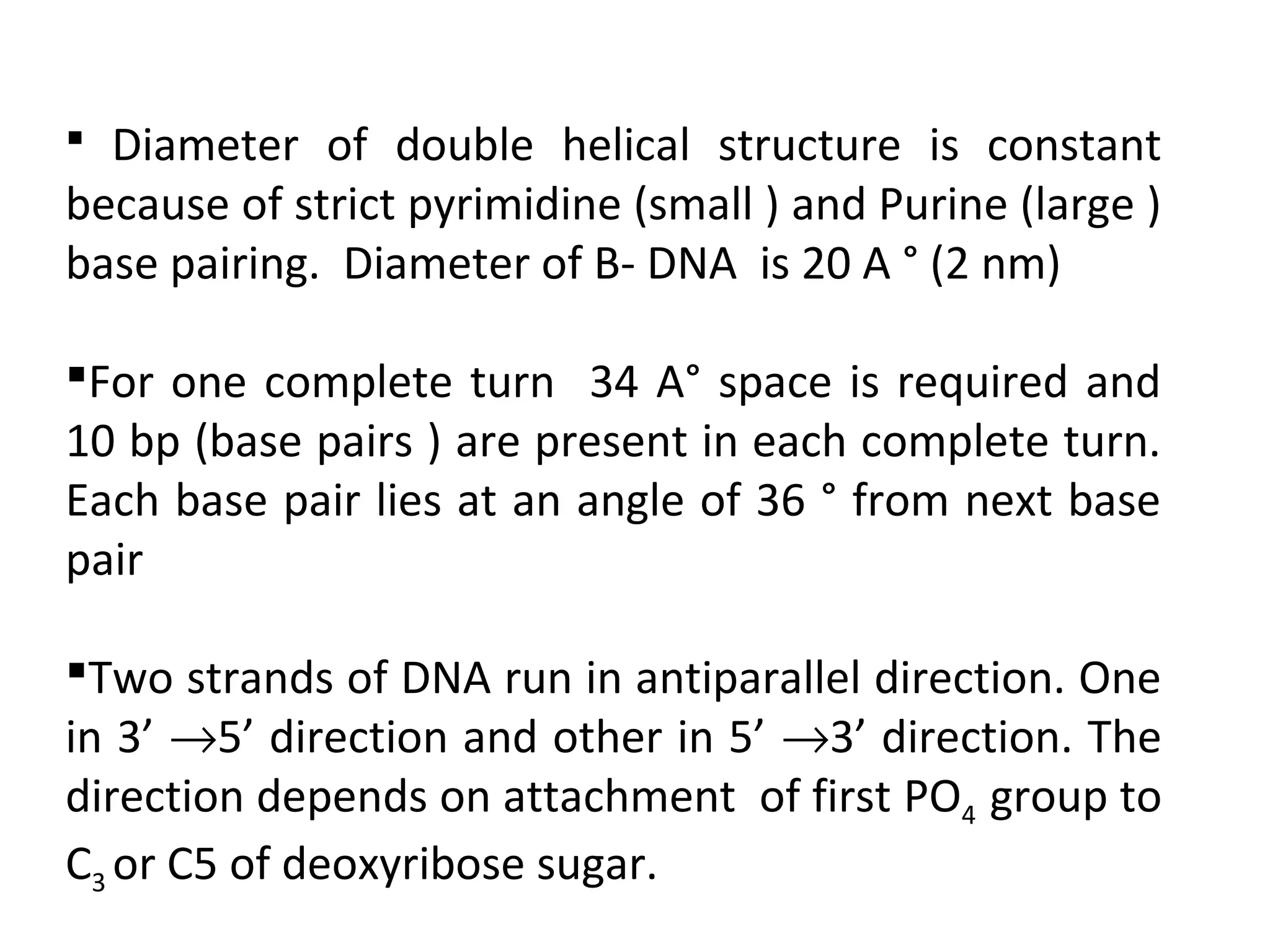
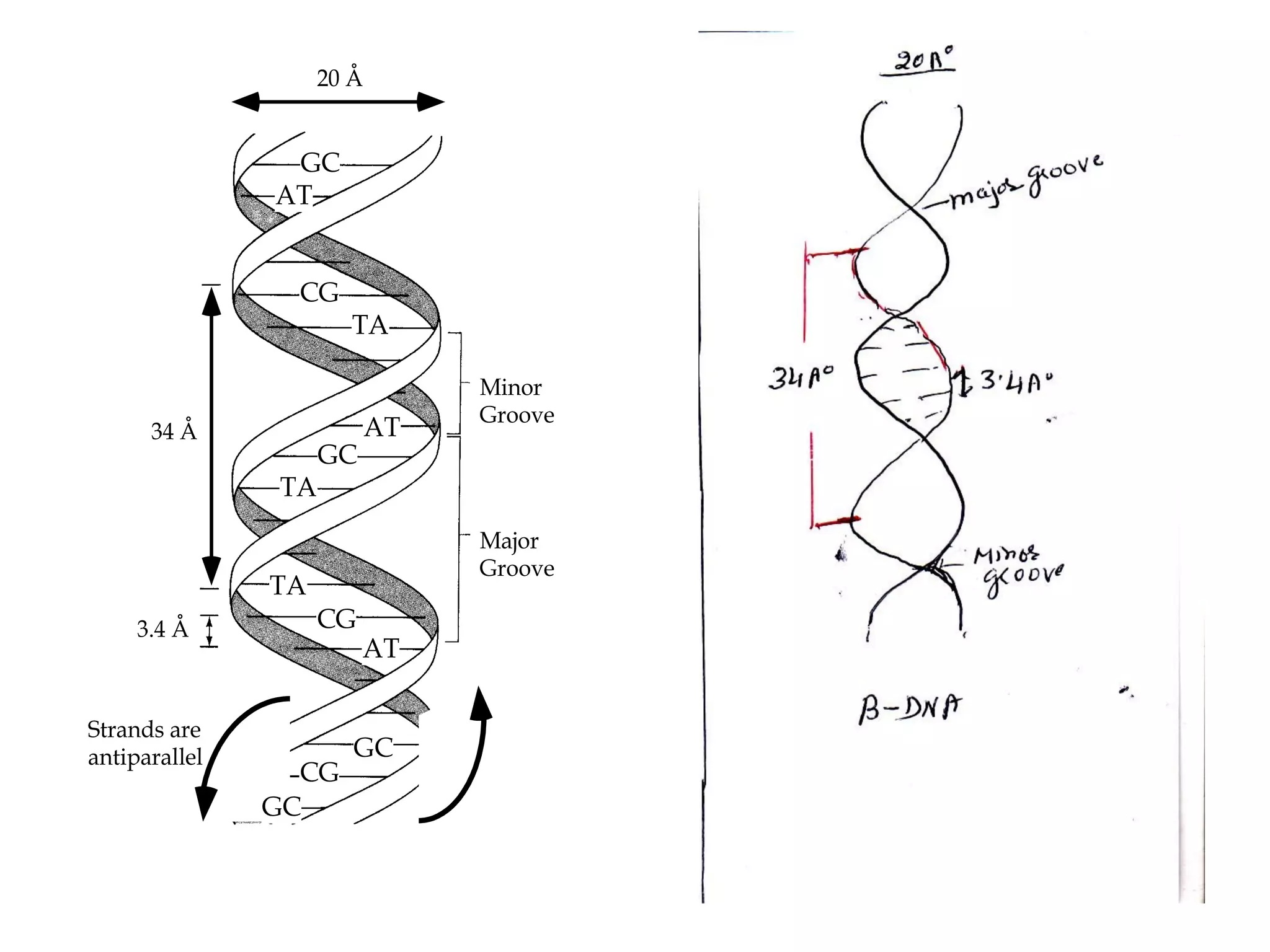
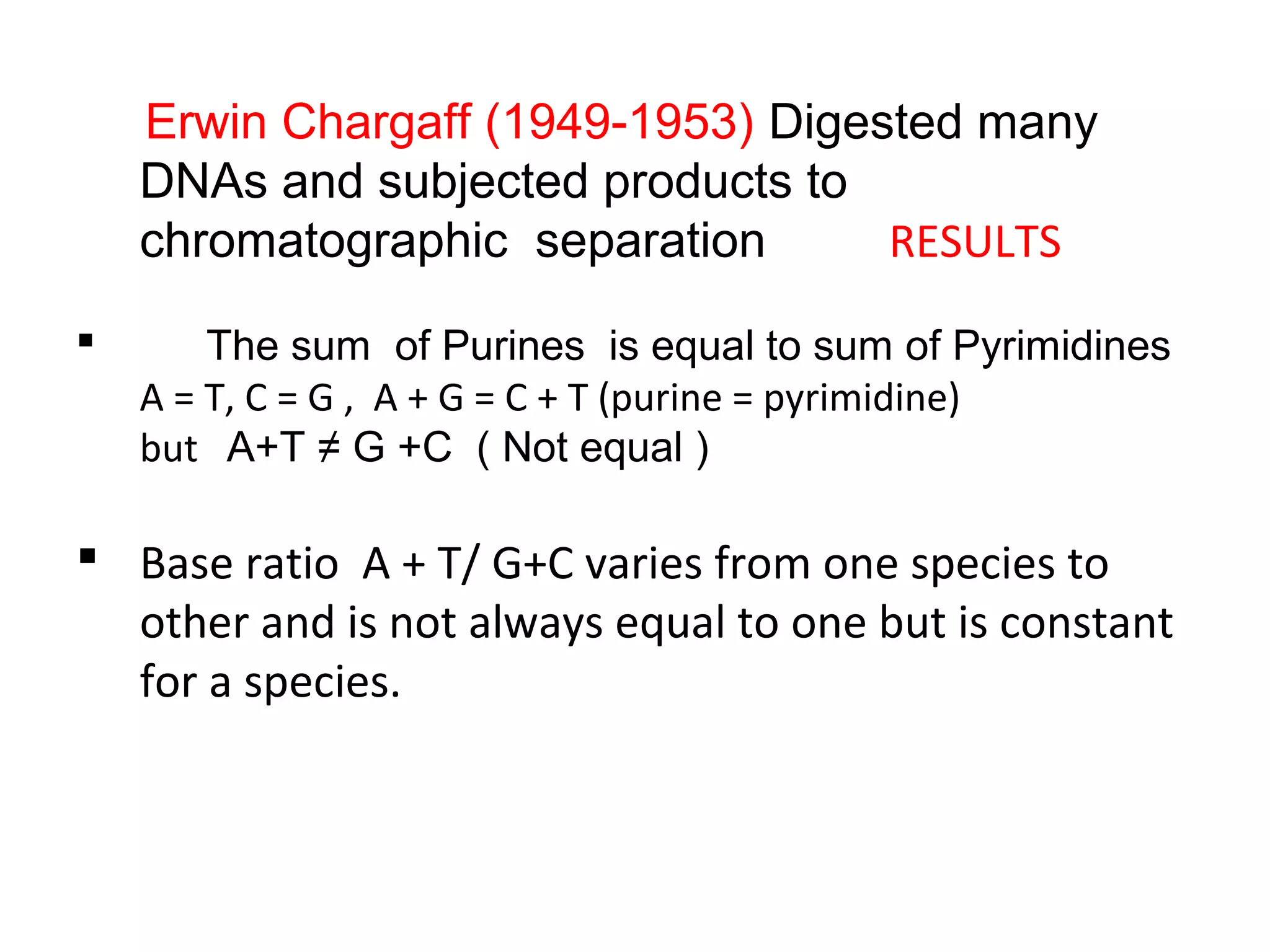
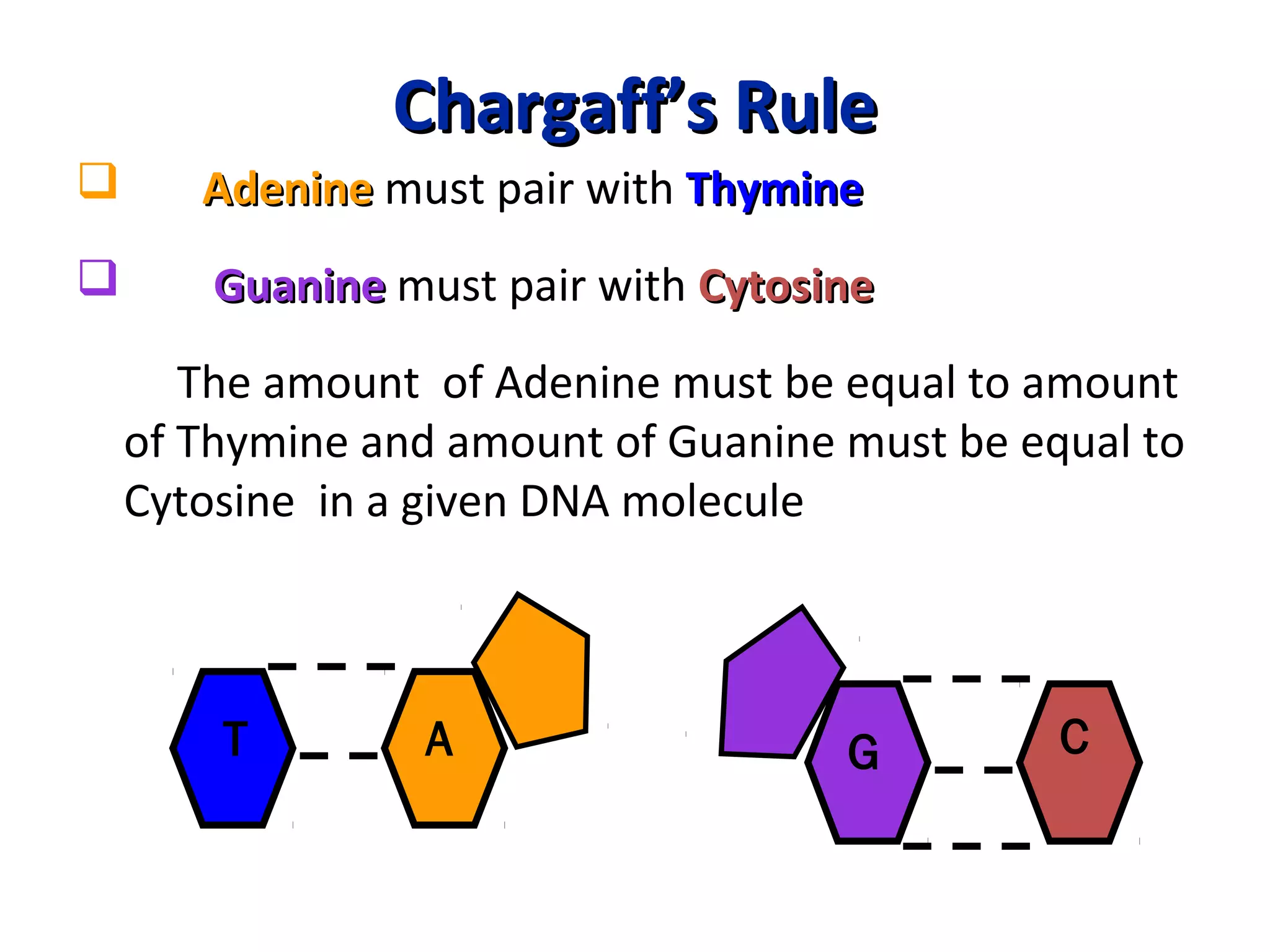
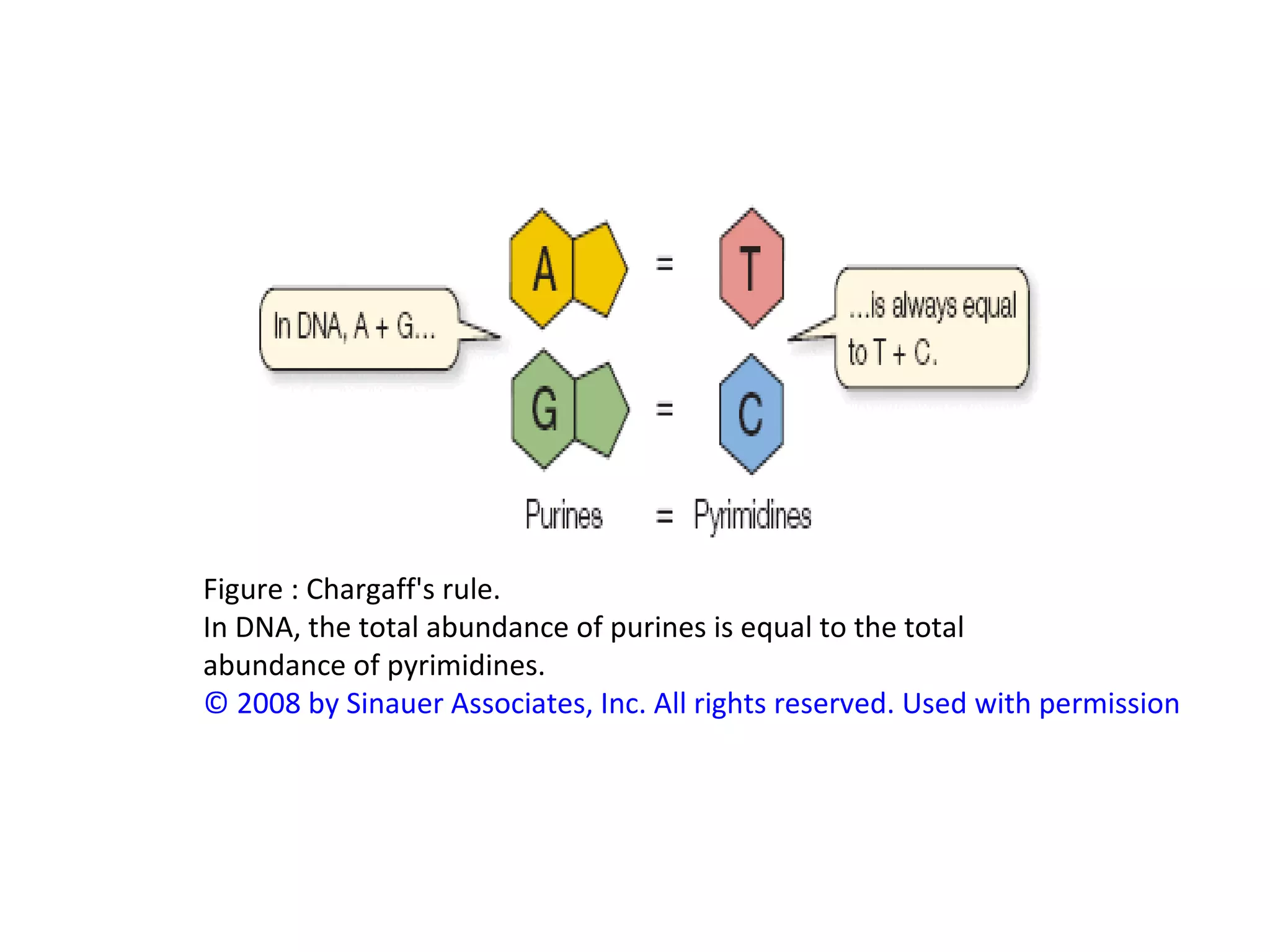
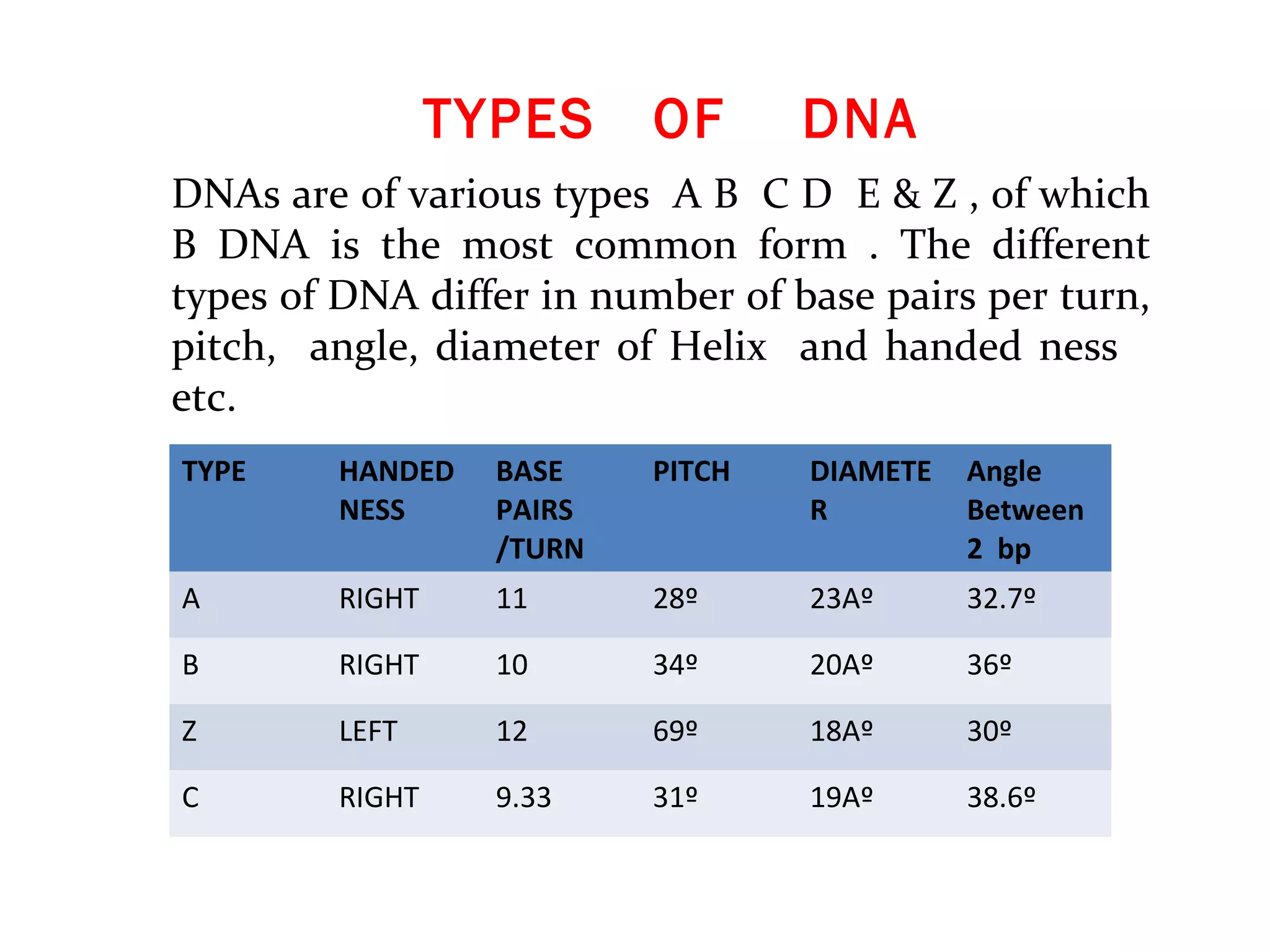
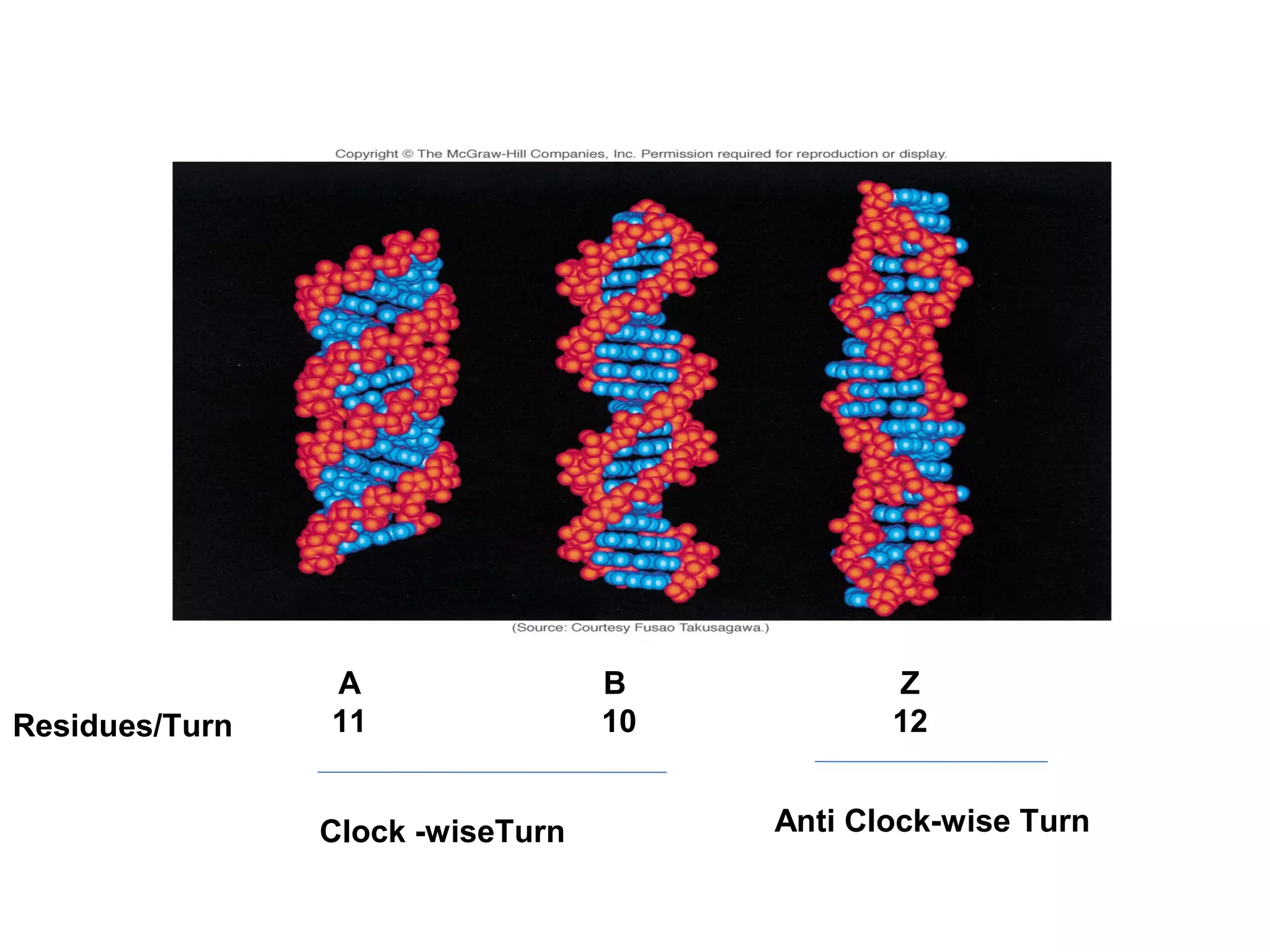
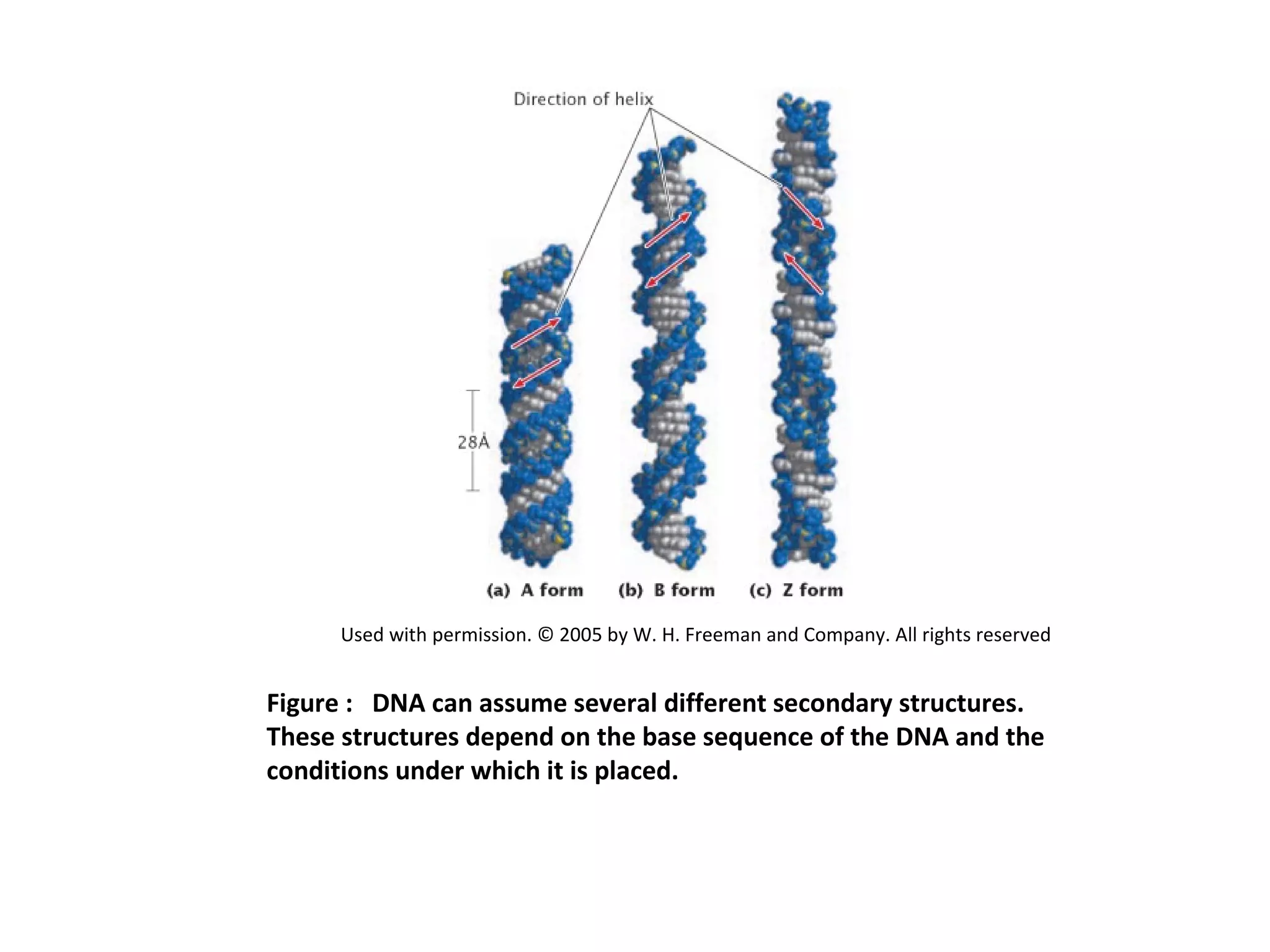
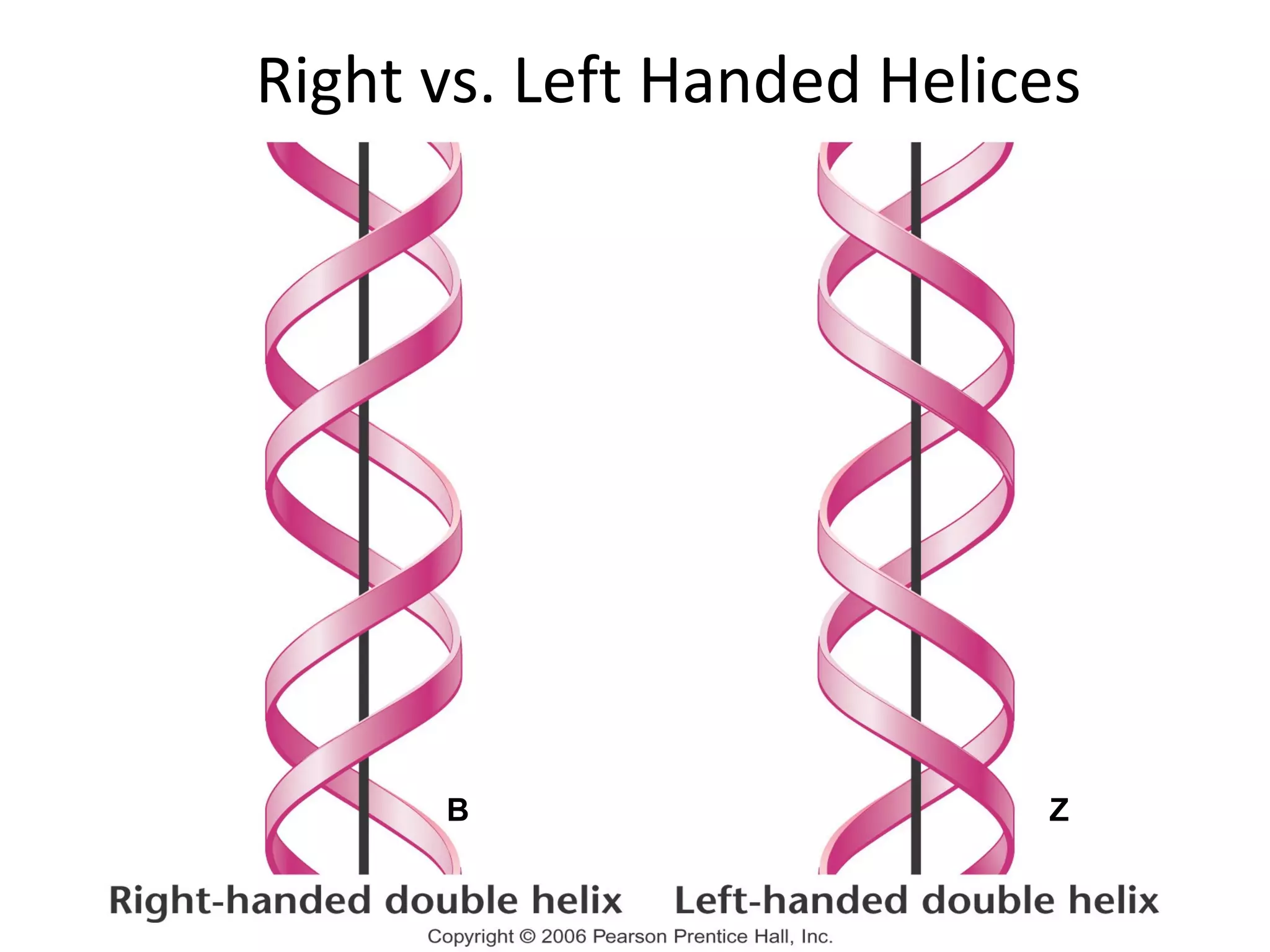
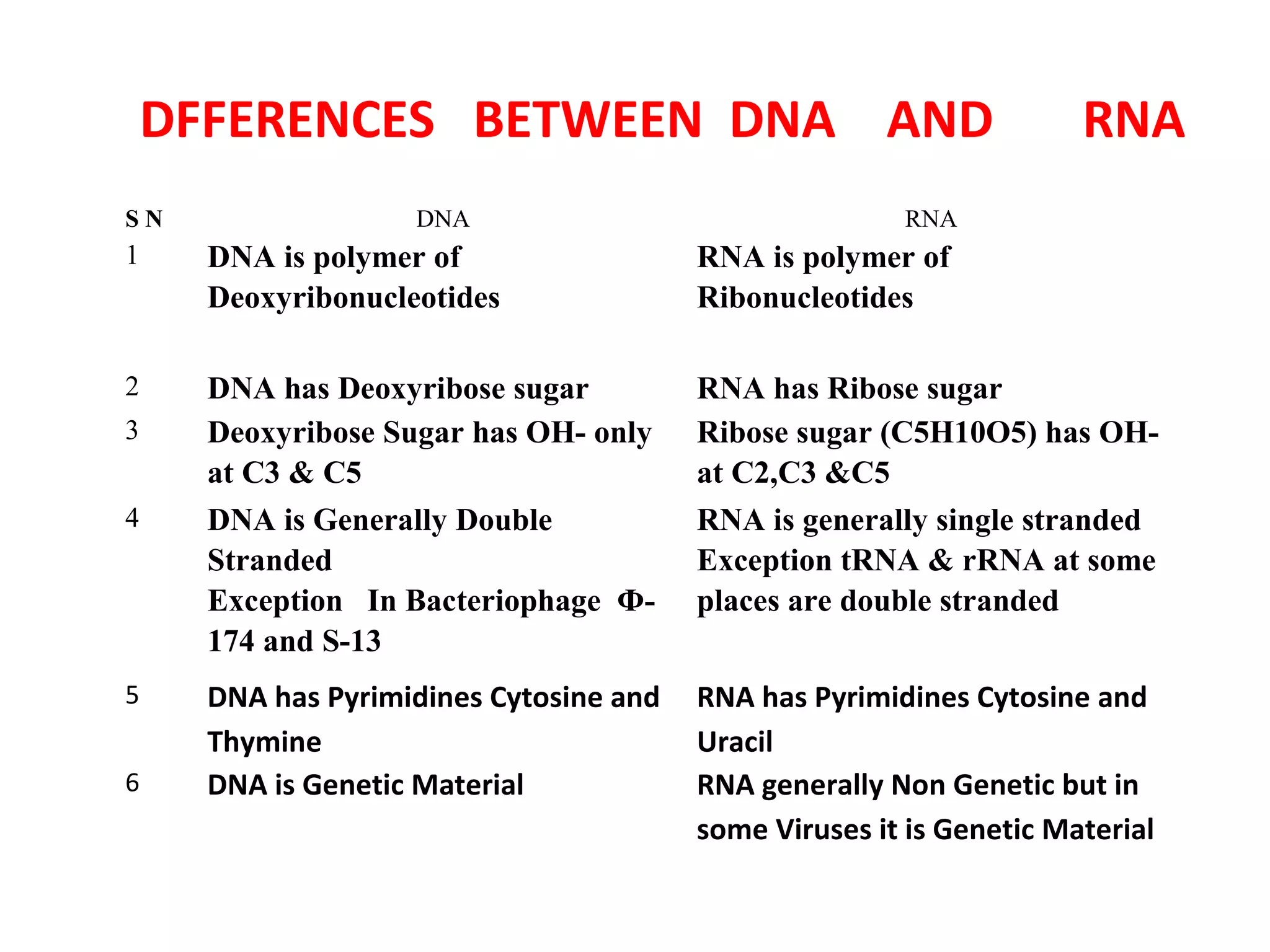
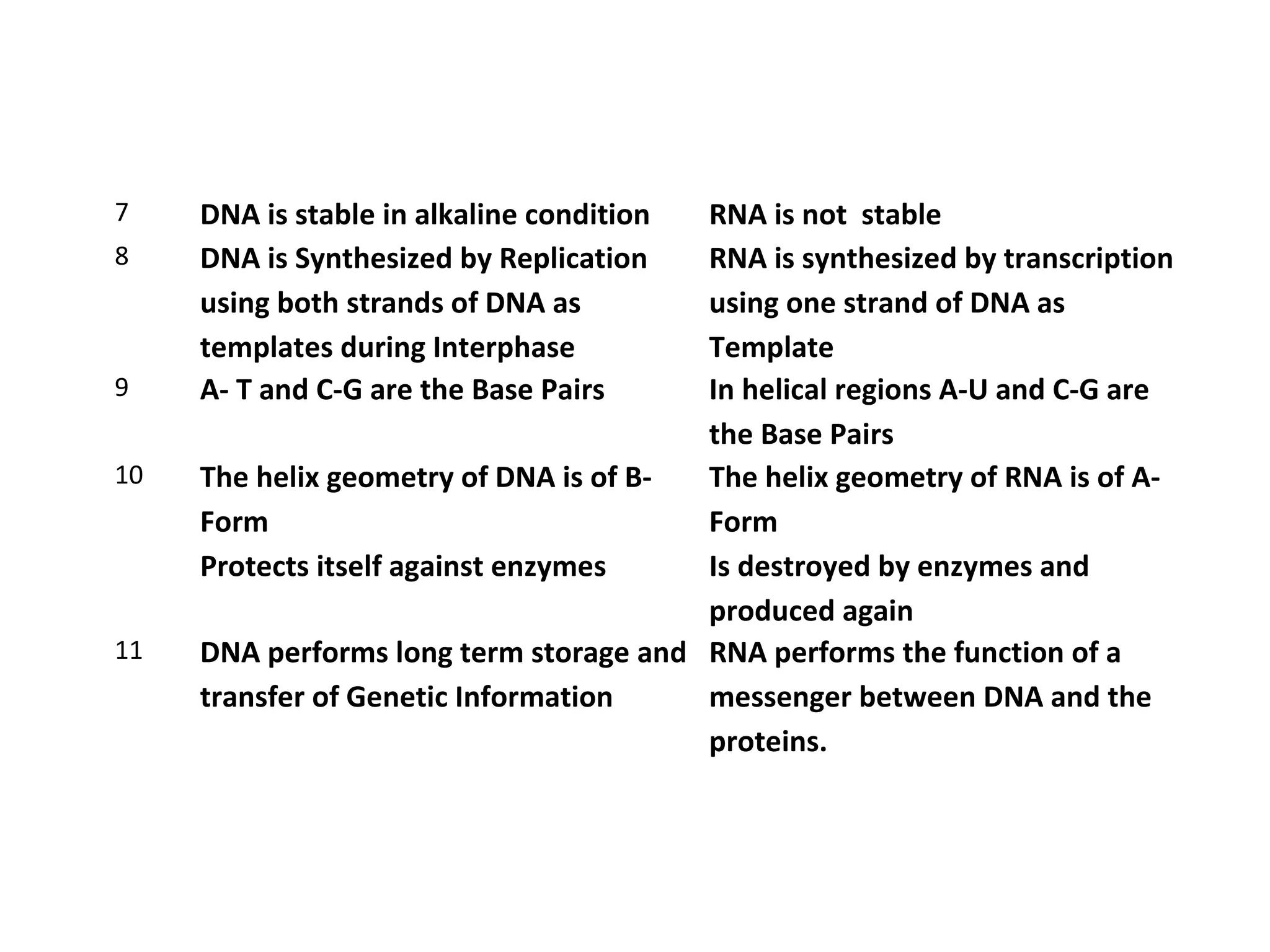
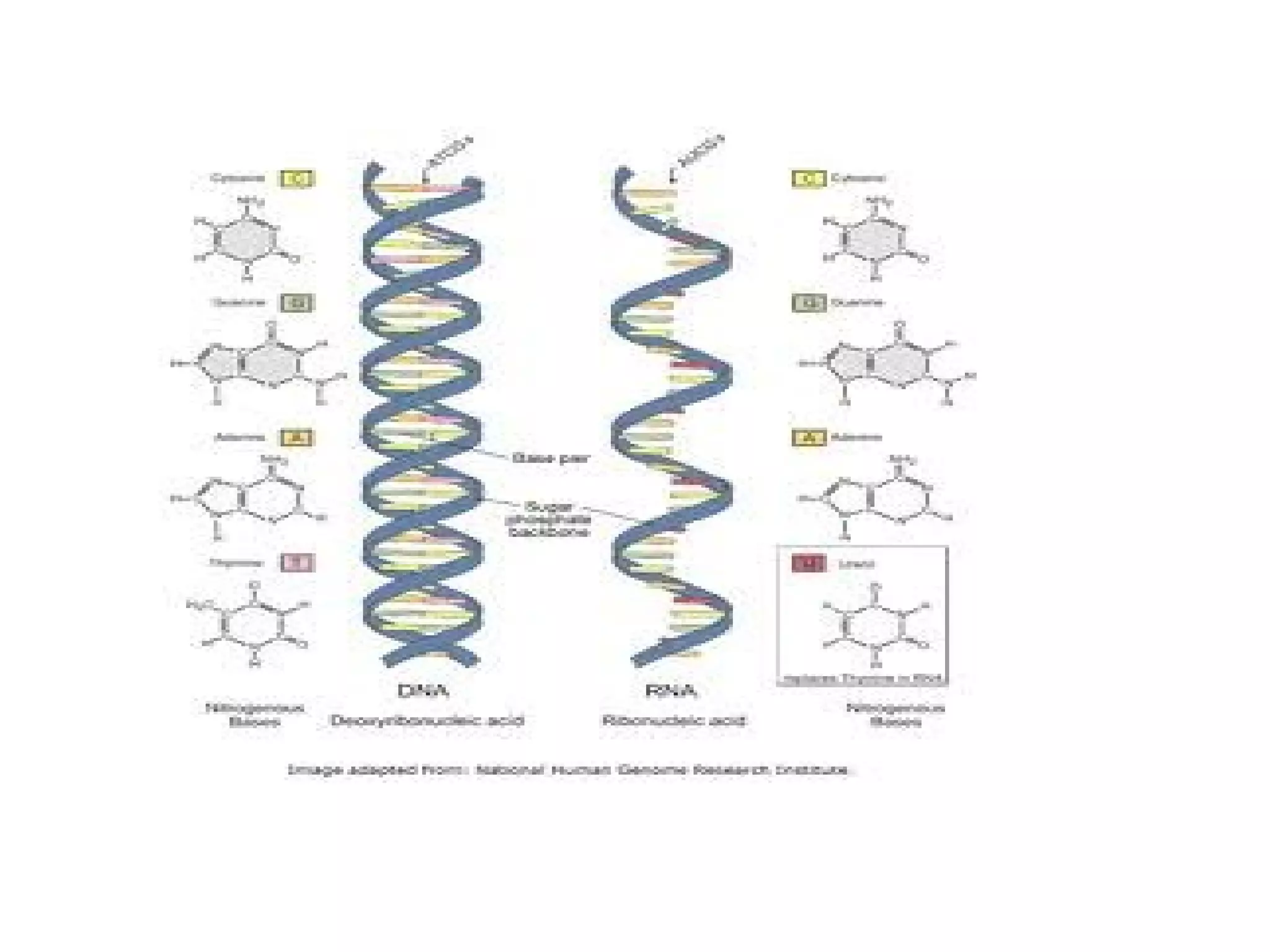
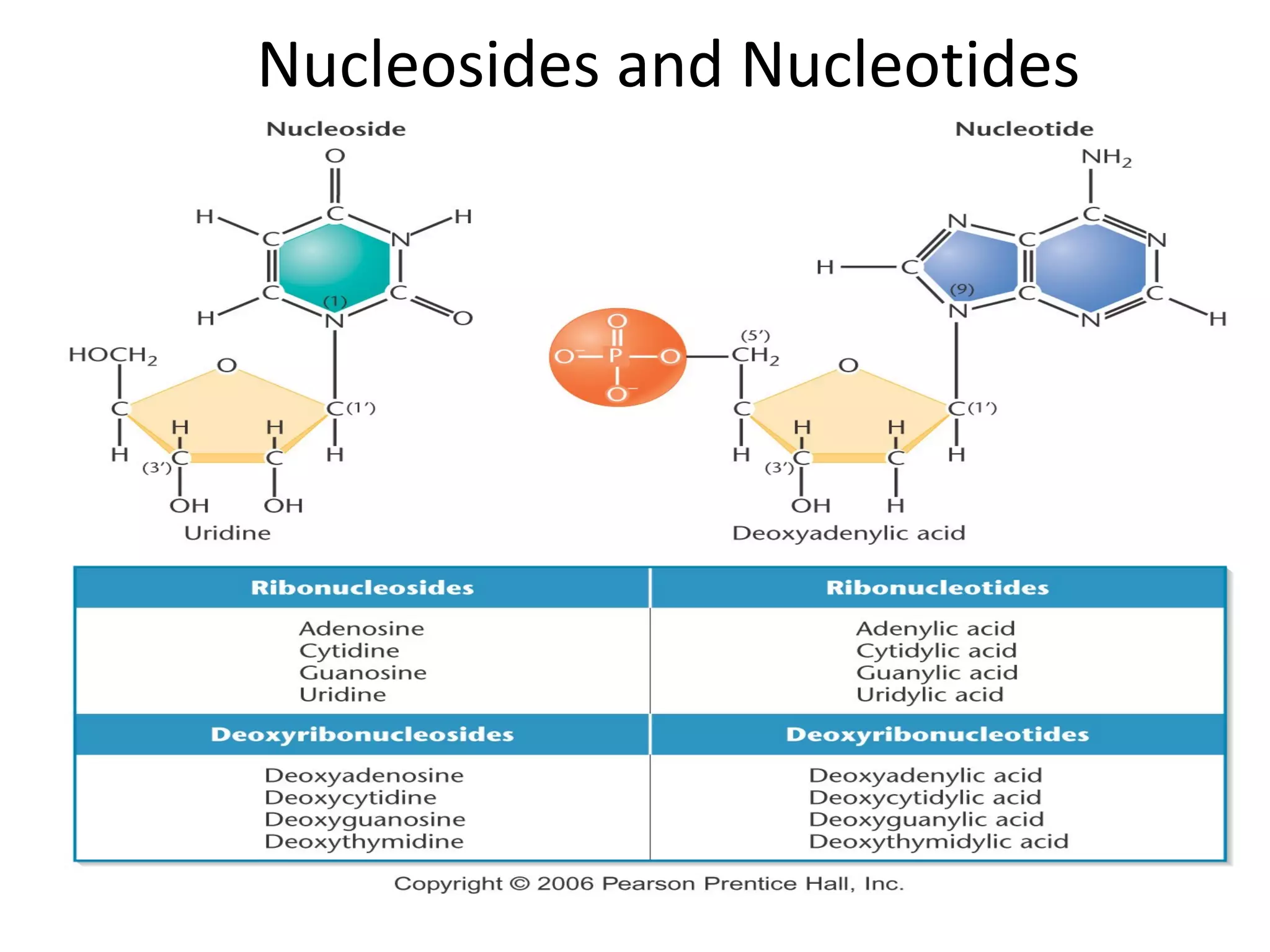
The document discusses the structure and discovery of DNA, detailing its components, helical structure, and the significance of base pairing in genetic replication and transcription. It outlines historical developments by various scientists including Watson, Crick, and Franklin, emphasizing Chargaff's rules regarding nucleotide pairing. Additionally, it compares DNA with RNA and highlights the stability and function of each type of nucleic acid.