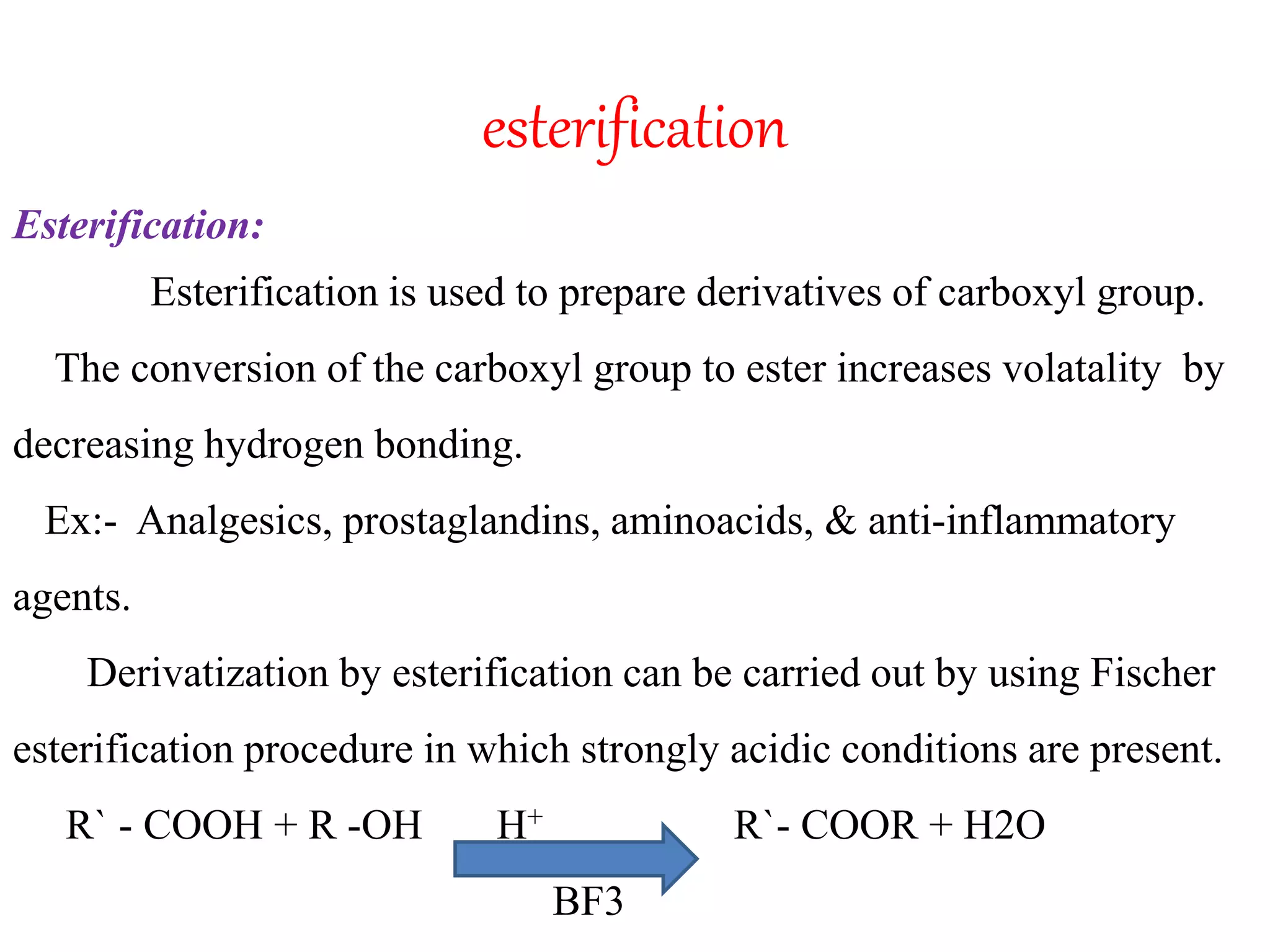
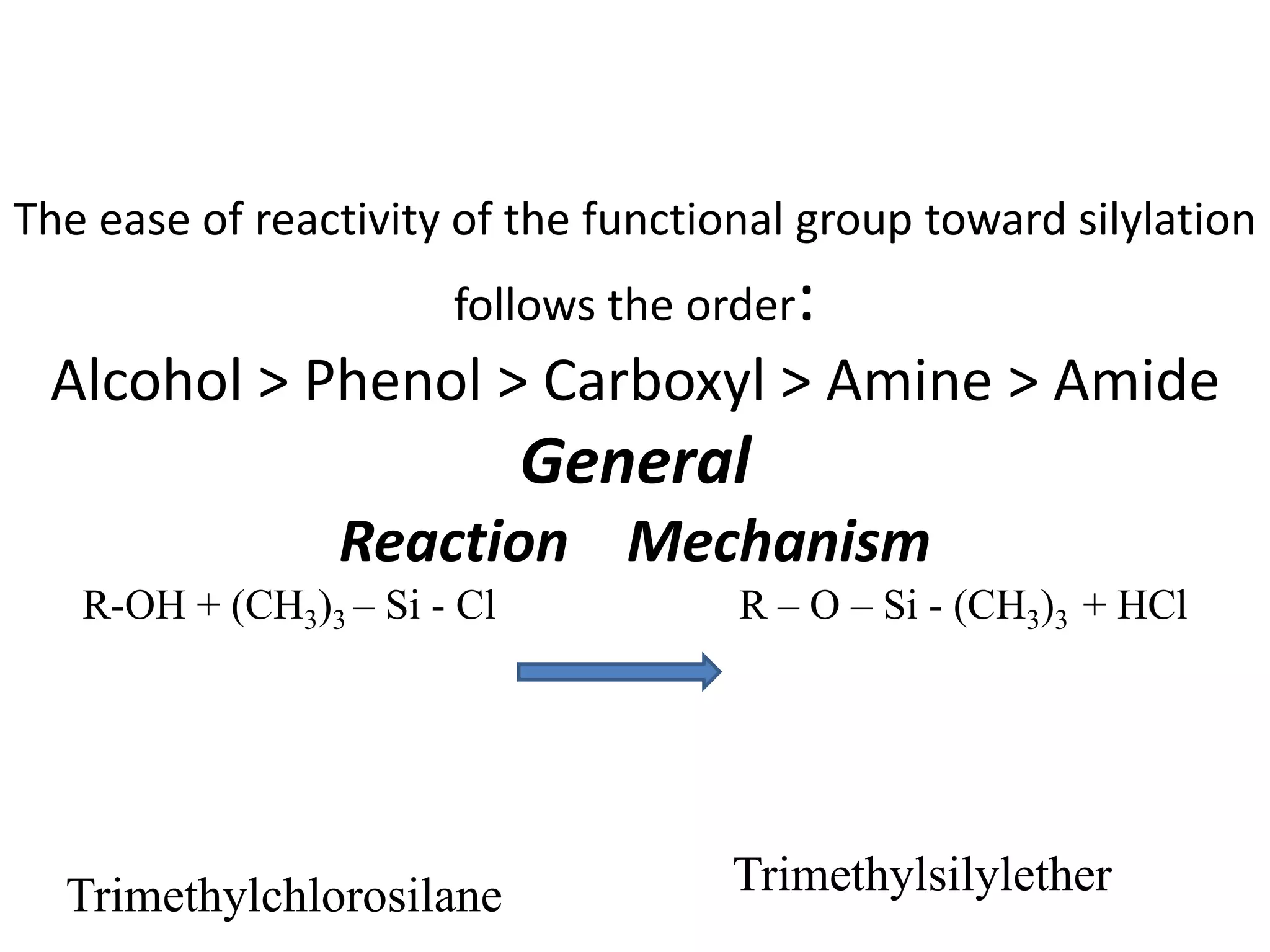
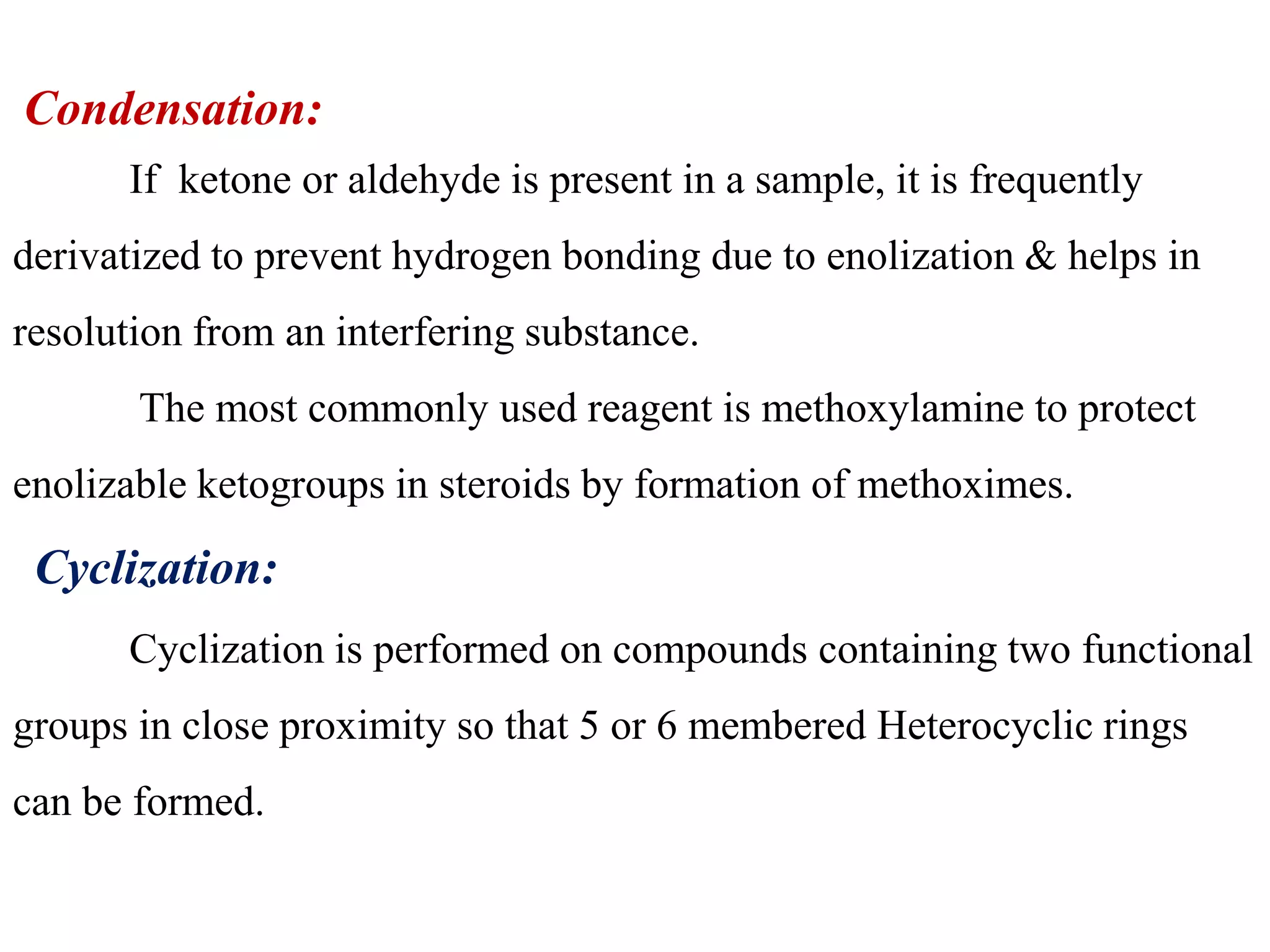
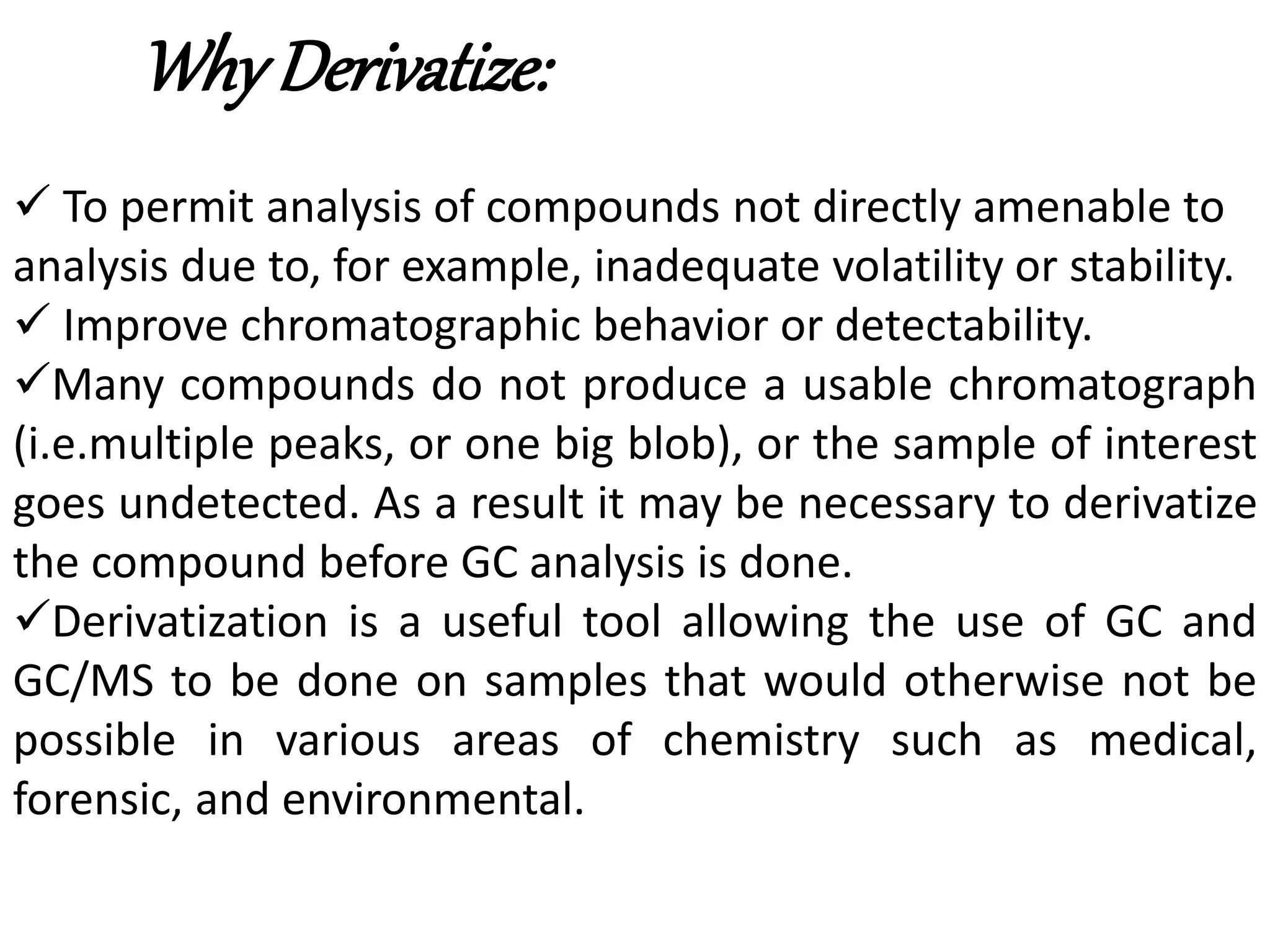
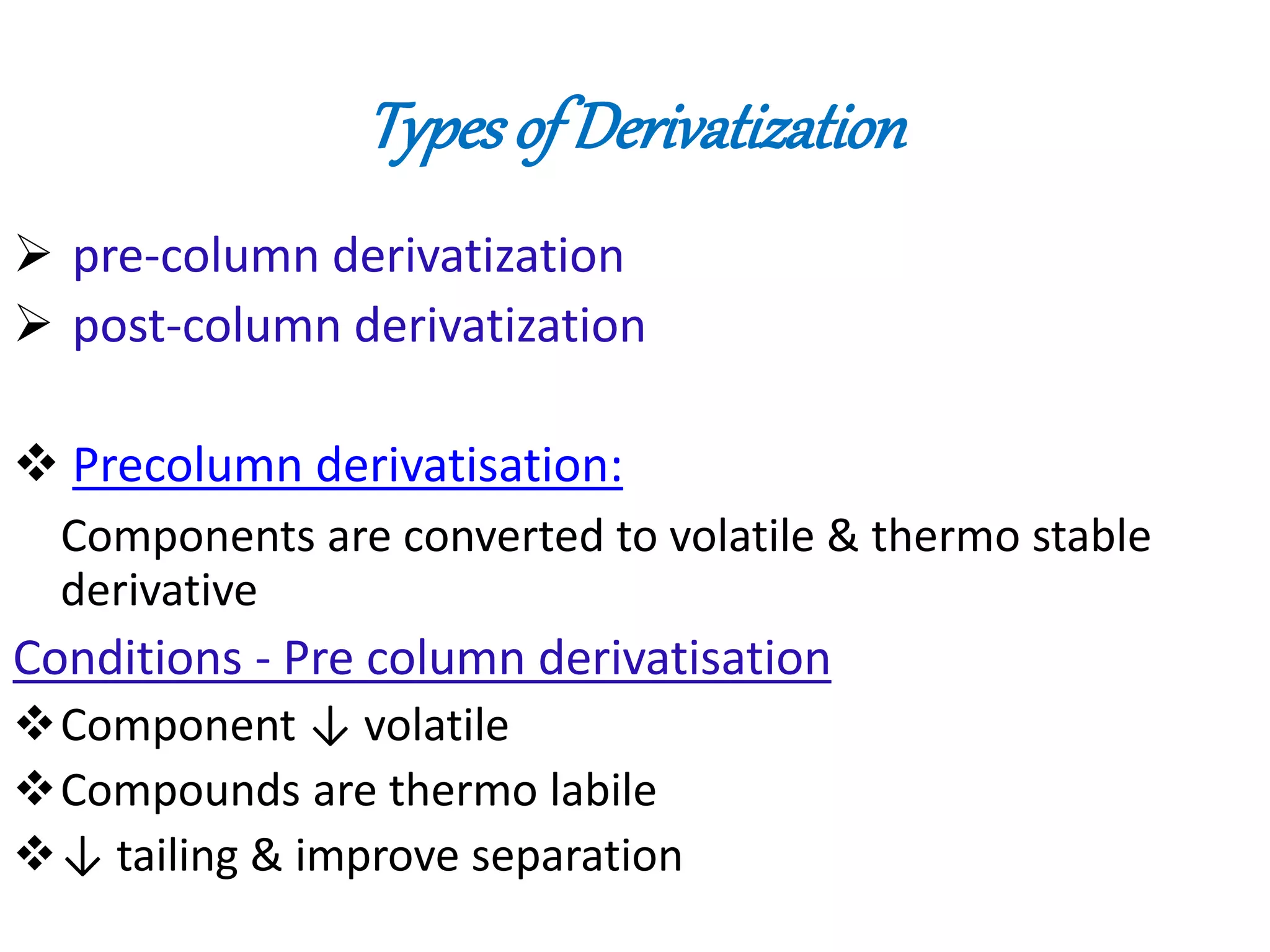
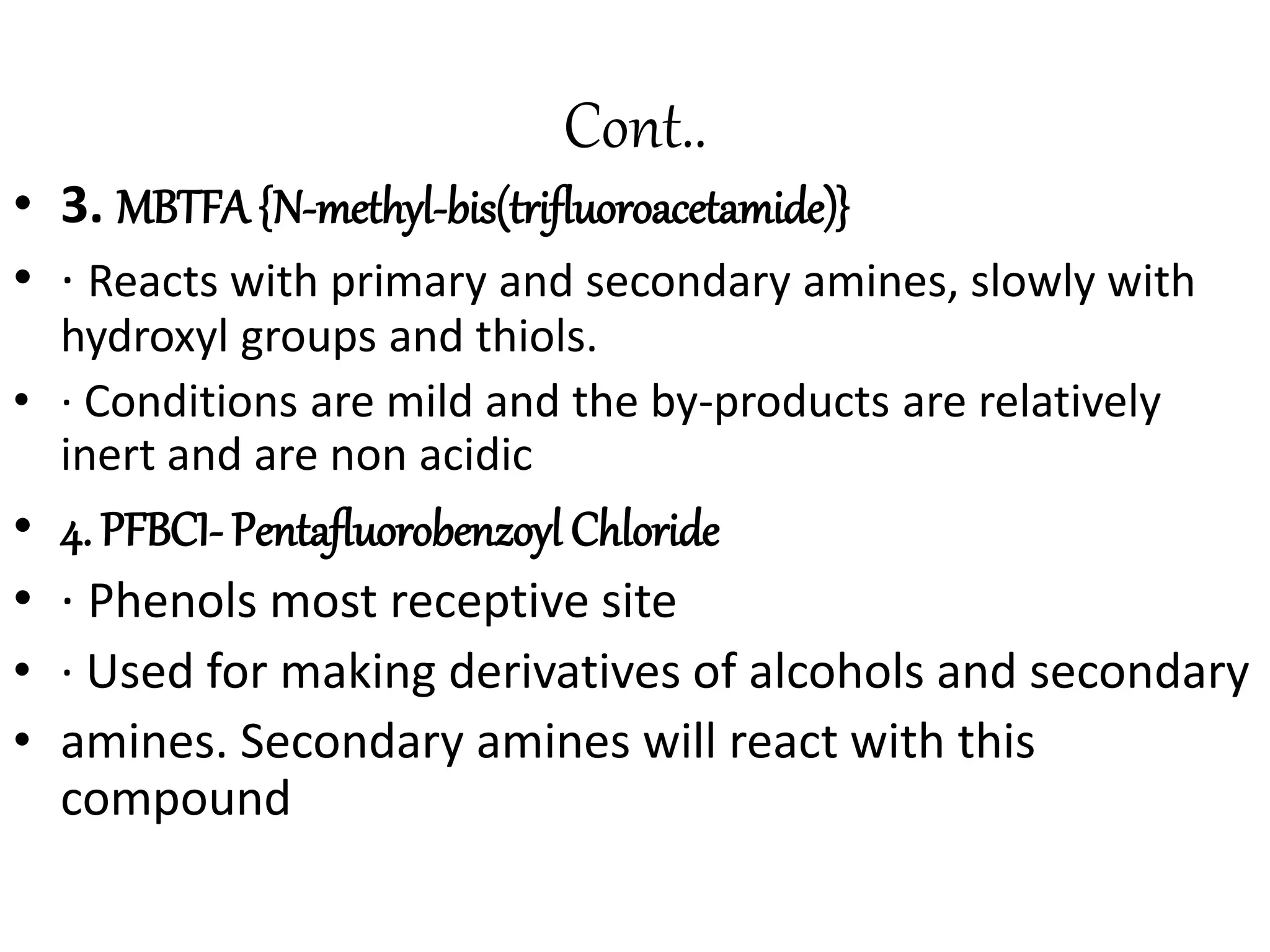
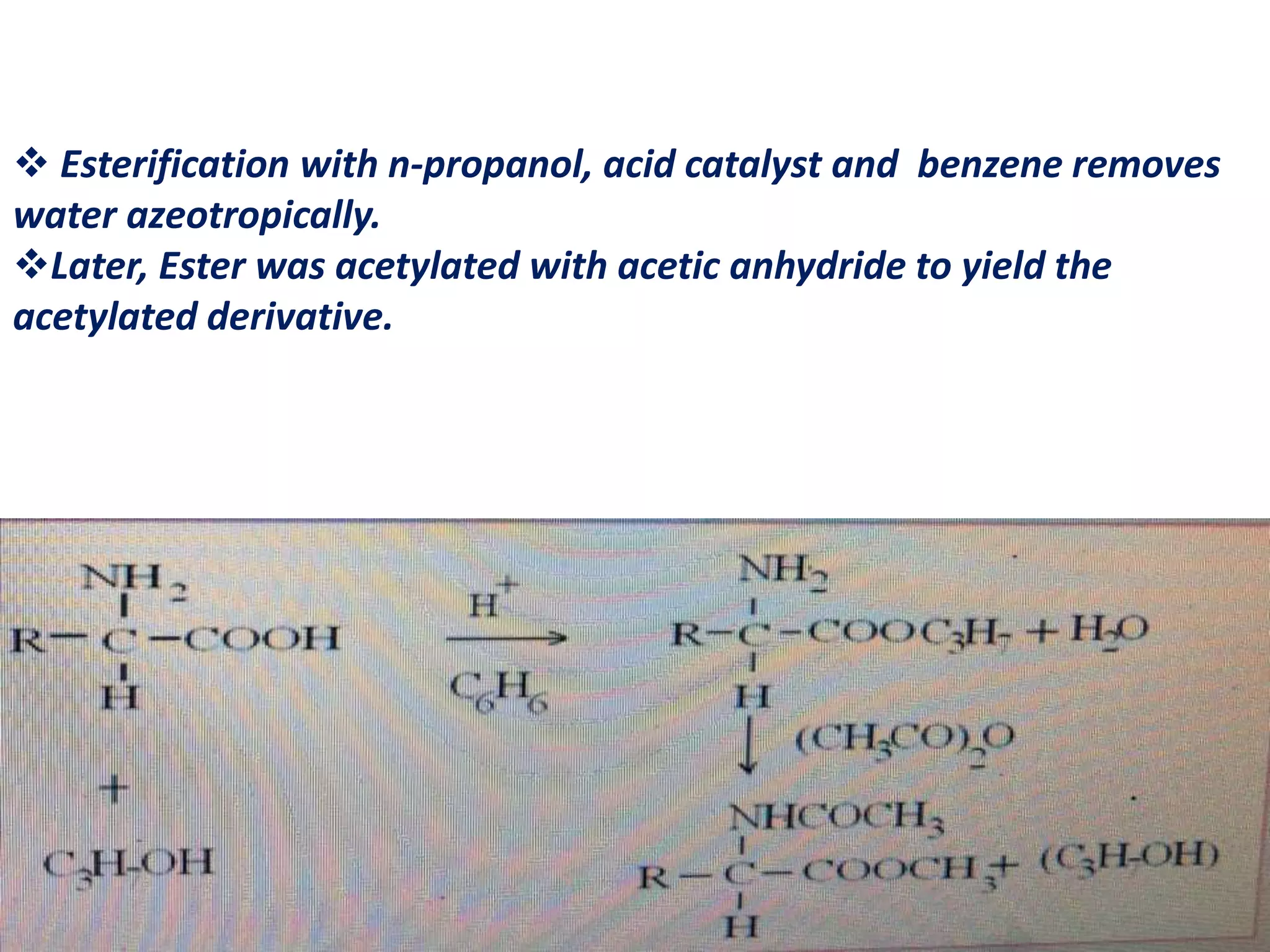
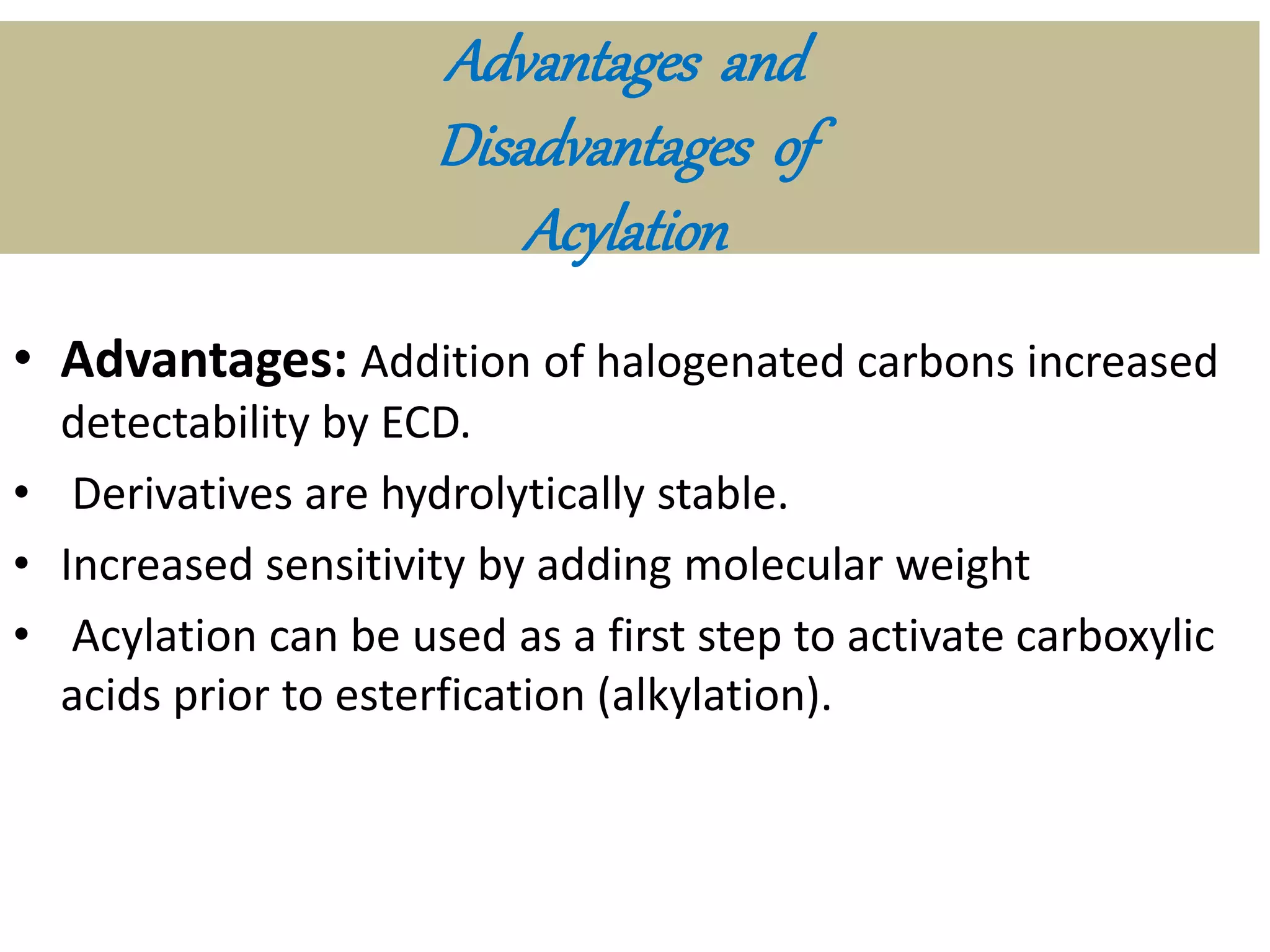
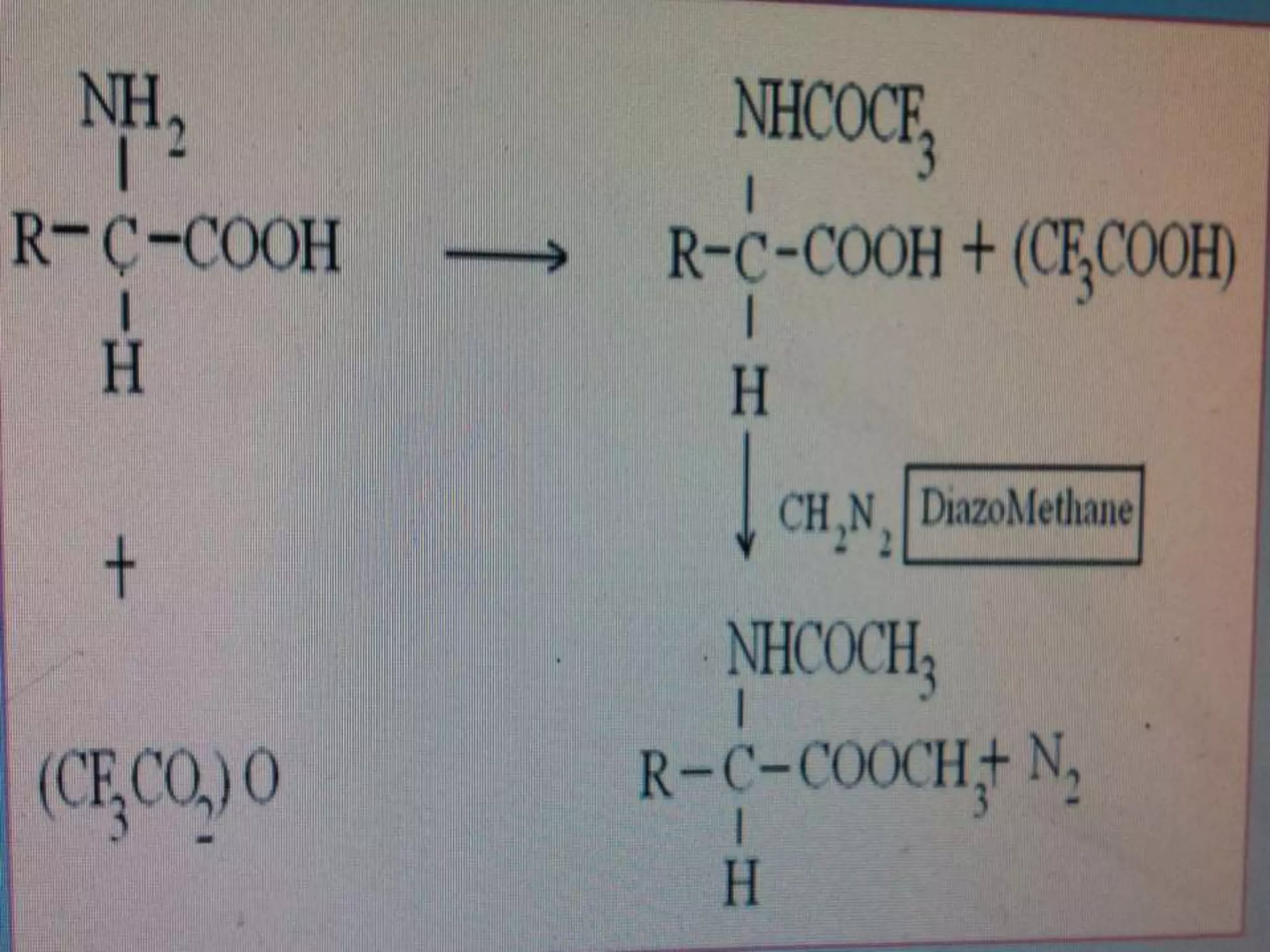
Derivatization is a process used to chemically modify compounds to make them suitable for gas chromatography analysis by increasing their volatility and thermal stability. It is commonly used to analyze compounds that are not directly amenable to GC due to issues like inadequate volatility, stability, or detectability. Common derivatization techniques include silylation, acylation, alkylation, and esterification. These techniques modify functional groups like hydroxyl, amino, and carboxyl groups to enhance chromatographic behavior and detectability.
















![·Eg: The imide nucleus is present in no. of pharmaceuticals
agents like anticonvulsants and barbiturates.
The most common derivative is methyl imide, which can be
formed on column by using trimethyl ammonium
hydroxide.[TMAH]](https://image.slidesharecdn.com/derivitizationofgc-140928235926-phpapp01/75/Derivitization-of-gc-24-2048.jpg)