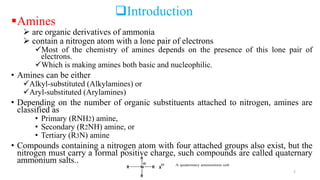
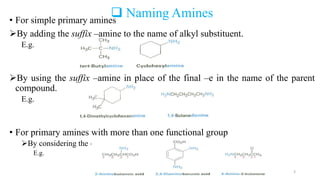
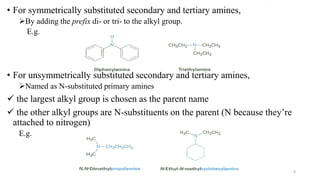
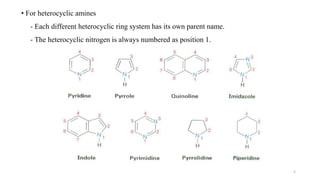
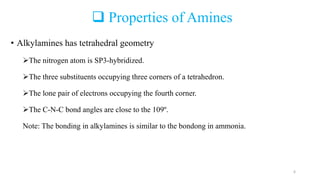
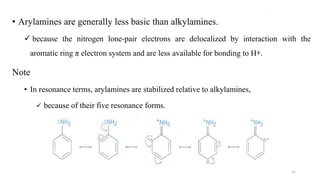
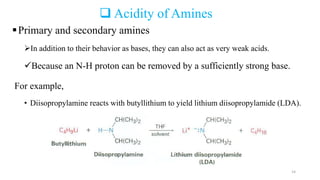
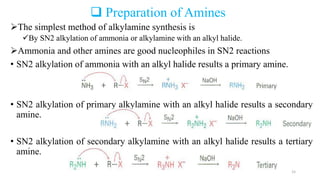
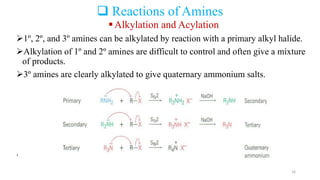
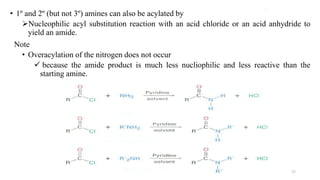
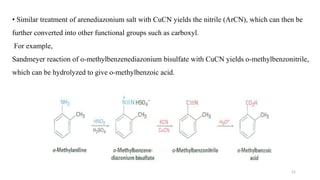
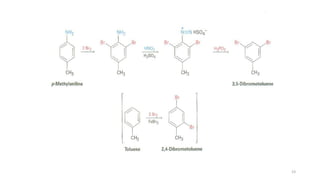
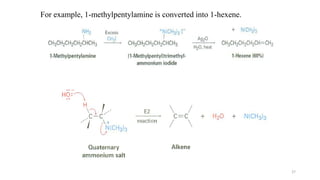
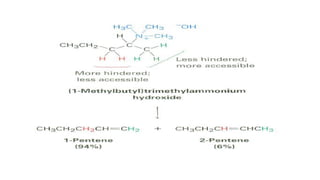
Amines are organic compounds containing a nitrogen atom with a lone pair of electrons. They can be classified as primary, secondary, or tertiary depending on the number of organic substituents attached to the nitrogen. Amines are named systematically and have basic properties due to the lone pair on the nitrogen. Common reactions of amines include alkylation, acylation, diazotization, and reduction of diazonium salts.