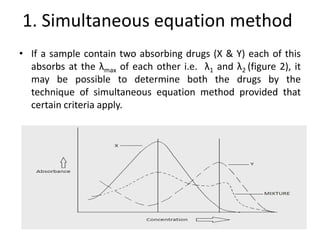
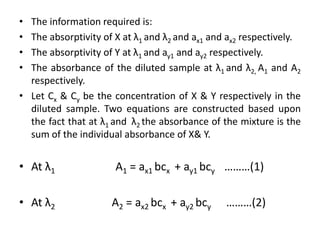
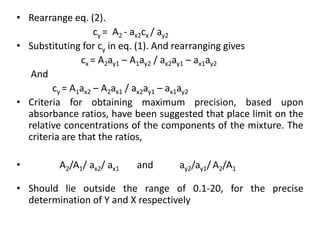
The document discusses various spectrophotometric methods for analyzing drug samples containing multiple absorbing components, including simultaneous equation method. The simultaneous equation method allows determination of concentration of two drugs (X and Y) that absorb at each other's wavelength maxima. It requires knowing the absorptivity of each drug at the two wavelengths and the sample absorbances at those wavelengths to set up two equations and solve for the unknown concentrations. Certain criteria on absorbance ratios must be met for precise results.