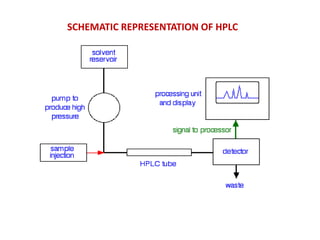
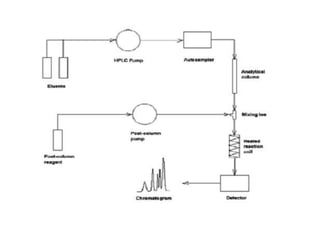
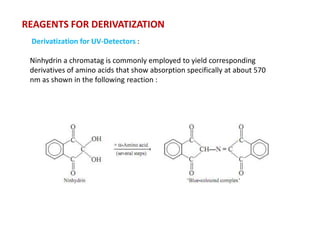
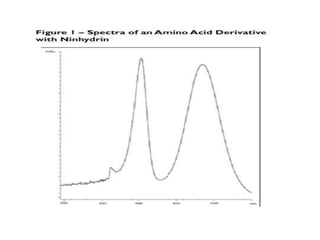
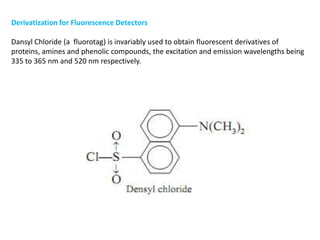
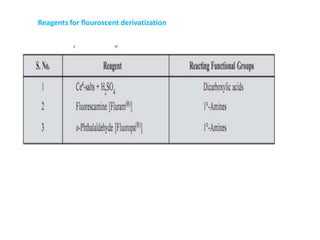
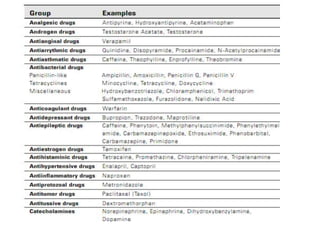
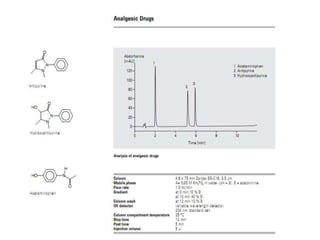
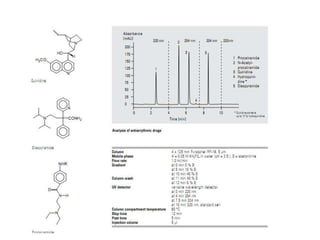
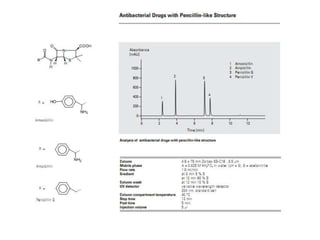
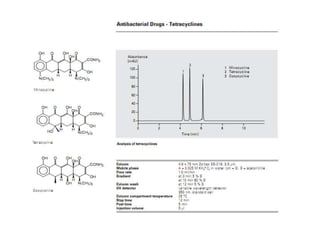
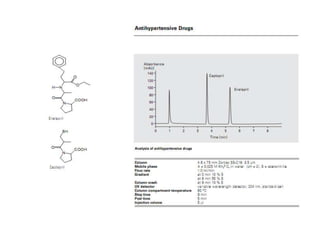
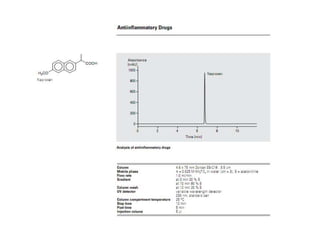
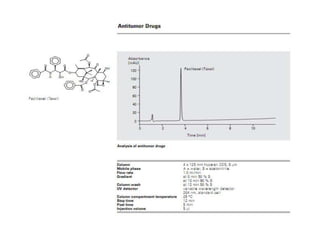
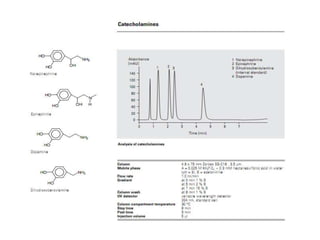
HPLC is a technique that separates mixtures by forcing a solvent through a column under high pressure. Derivatization transforms compounds into derivatives with different properties to improve separation and detection. It can occur before or during HPLC. Pre-column derivatization changes properties before injection while post-column occurs on a reactor between the column and detector. Reagents are chosen to yield UV or fluorescence suitable for different detectors. HPLC and derivatization are useful for drug manufacturing control and analysis.