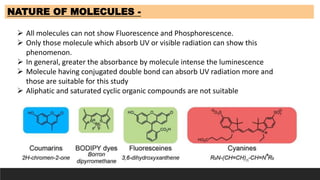
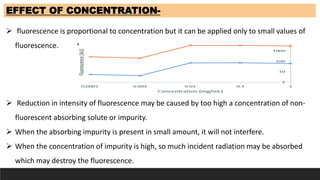
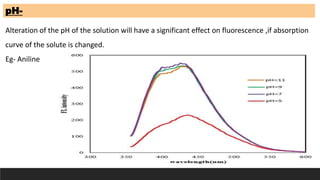
The document discusses various factors influencing fluorescence and phosphorescence, such as molecular nature, substituent effects, concentration, and environmental conditions like temperature and pH. It highlights that certain molecules are capable of luminescence, and specific applications of fluorimetry include vitamin analysis and organic compound detection. Key points emphasize the role of electron-donating and withdrawing groups on luminescence and the importance of using monochromatic light for excitation.