Dental Products Manik
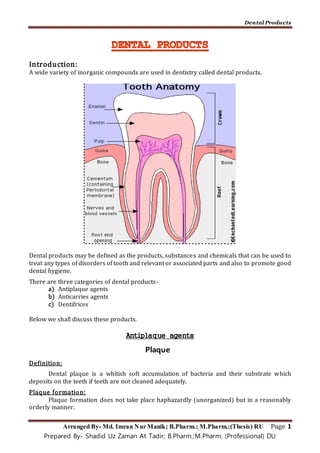
- 1. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 1 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU Introduction: A wide variety of inorganic compounds are used in dentistry called dental products. Dental products may be defined as the products, substances and chemicals that can be used to treat any types of disorders of tooth and relevant or associated parts and also to promote good dental hygiene. There are three categories of dental products- a) Antiplaque agents b) Anticarries agents c) Dentifrices Below we shall discuss these products. Antiplaque agents Plaque Definition: Dental plaque is a whitish soft accumulation of bacteria and their substrate which deposits on the teeth if teeth are not cleaned adequately. Plaque formation: Plaque formation does not take place haphazardly (unorganized) but in a reasonably orderly manner.
- 2. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 2 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU 1. Formation of pellicle: Pellicle is a thin, clear cuticle composed of glycoproteins that is formed from saliva and gingival fluid. It is formed first on the tooth. Formation of pellicle occurs specially after intake of food. 2. Attachment of bacteria: Very soon after pellicle formation, bacteria of the coccus, type (largely streptococci) are attached to the pellicle which has a sticky surface. This stickiness enables colonies of organisms to be anchored. These organisms divide and form colonies. 3. Attachment of other microbes: Attachment of the microorganisms is further enhanced by the production of dextrans by the bacteria as by-products of metabolic activity. Later other organisms are attached to the mass and a dense mixed flora of filamentous forms i.e. plaque result. Where is plaque formed? Plaque is attached to the teeth supragingivally (in the supragingival region – above the gingival area i.e. the gum of the tooth) or subgingivally (beneath the gingiva) in the gingival crevice (crack) and primordial pockets. Antiplaque agents Antiplaque agents are the agents or drugs that are used to prevent or inhibit plaque formation in the mouth. Such agents are chlorhexidine and povidone-iodine. Ideal properties of an antiplaque agent: 1. It should be non-toxic, non-allergic and non-irritating. 2. It should be a broad spectrum of antimicrobial activity. 3. It should only specifically affect the pathogenic flora. 4. It should not have any induced drug resistance. 5. It should have an acceptable taste. 6. It should possess sufficient chemical stability. So that it can be stored for a reasonable length of time. 7. It should be of low price and available. Chlorhexidine: Chemistry: It is chemically chlorphenyl bisbiguanide. H N H N NH (CH2)6 H N H N H N NH NH NH NH Cl Cl Chlorpheyl bisbiguanide
- 3. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 3 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU It is available as acetate or most commonly as gluconate salt in mouth rinses, gels or dentifrice for the control of plaque and gingivitis. Mechanism of action: Due to its high cationic nature chlorhexidine binds to the anionic groups on the bacterial surface, i.e. phosphate group of teichoic acid in gram-positive bacteria and phosphate group of lipopolysaccharides in gram negative bacteria. When the bisbiguanides binds to the organism, the cell membrane becomes permeable allowing the cytoplasmic contents to leak out of the cell. At higher concentration chlorhexidine also causes precipitation of cytoplasmic proteins. By virtue of the cationic properties of the bisbiguanides also bind electrostatically to the hydroxy-apatite of the teeth and forms pellicle (film or surface) against plaque and to buccal mucosa. All these activity altogether contribute to its action as an antiplaque agent. Use: A. It is highly cationic antiseptic and has fungicidal activities as well as bactericidal activities against both gram (+) and gram (-) bacteria. B. It is used to control plaque and gingivitis in following products- a. Mouth rinses: 0.12% chlorhexidine gluconate is used as a mouthwash for oral hygiene and oropharyngeal infections especially aphthous ulcers. 0.2% chlorhexidine gluconate solution prevents the accumulation of plaque. It absorbs onto the tooth enamel where it exerts a persisting action to decrease the growth of dental plaque. b. In irrigations: 400 mL of 0.02% of chlorhexidine (80 mg totally) applied once daily in an oral irrigator will give complete plaque inhibition. c. Gels: 1% gel of chlorhexidine gluconate applied for a period of five minutes, once or twice daily is effective in the inhibition of bacterial plaque particularly in the treatment of denture stomatitis (inflammation of the oral mucosa in contact with the denture). d. Antiseptics: 4% aqueous solution of chlorhexidine is effectively used as a surgical scrub (to clean the patient’s skin). It decreases the cutaneous bacterial population more than hexachlorophene or povidone-iodine. Side-effects of chlorhexidine: i. The most common side-effect of chlorhexidine is the staining of the teeth or formation of yellowish or brownish discoloration of the tooth surface and the gum. Stain may appear on the natural teeth, artificial teeth and composite filling though it depends on the concentration and varies greatly from one individual to another. ii. It has unpleasant bitter taste which causes irritation of the oral mucosa and disturbances in the taste buds. iii. Regular use of chlorhexidine sometimes blocks the salivary duct of the parotid gland and causes painful condition. iv. Local tissue damage may occur if it is applied to abraded (torn by friction) epithelium.
- 4. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 4 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU v. Hypersensitivity reaction may occur in some individuals. Precautions: i. Chlorhexidine rinses should be performed after meals to minimize taste alteration. ii. Patients should not rinse with water after chlorhexidine rinsing. Povidone-Iodine: Chemistry: Povidone-iodine is a stable complex of Polyvinylpyrrolidine (PVP) and elemental iodine. Why povidone-iodine is superior to conventional/classic iodine solutions: Povidone-iodine and simple iodine solutions give similar actions. But povidone-iodine is superior as- 1. It has low systemic toxicity. 2. Unlike iodine solutions, it doesn’t cause pain when applied to abraded tissue, wounds or mucous membrane. 3. It is less irritating. 4. Doesn’t permanently causes staining of the skin or hard surfaces. 5. The possibility of resistance by the microbes is less. 6. Effective as dilute solution. Use: 1. It is a broad spectrum germicide similar to chlorhexidine and also a bactericide effective against both gram (+) and gram (-) bacteria. 2. It is used in mouth washes and gargles for the treatment of acute mucosal infections of the mouth and the pharynx. 3. It is also used for oral hygiene prior, during or after oral surgery. Anticarries agent: Dental carries: Definition: Dental caries is gradual decay and disintegration of tooth tissues, i.e. progressive decalcification of the enamel and dentin of a tooth.
- 5. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 5 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU The ultimate effect of caries is to break down enamel and dentin and thus open a path for bacteria to reach the pulp. The consequences are inflammation of the pulp and, later, periapical tissues. Infection can spread from the periapical to the jaw and beyond. Carriesformation: Carries formation starts at the enamel of the tooth. It is initiated by the microorganisms which are in intimate relationship with the tooth surface. i. Form the metabolic activities of the microbes in the plaque acid is formed. These microbes act on the sucrose products entering the plaque after carbohydrate ingestion and form acids which at critical pH (below 5.5) cause damage to the enamel and hydroxyapatite is dissolved. ii. The decay penetrates the dentin. iii. Finally the carries communicates with the pulp. Types of dental carries: According to the location dental carries are i. Pits and fissure (the part where the tooth is broken) caries ii. Smooth surface caries iii. Root caries iv. Deep dentinal caries Factors involvedin the initiation of carries formation/essential requirements of carries formation:
- 6. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 6 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU Dental caries is a multifactorial disease and the following four factors are invo1ved in the initiation of dental caries- 1. Susceptible tooth surface to acid attack: Generally caries is initiated in the enamel but it may also begin in dentin or cementum. 2. Plaque attached to the tooth surface: Plaque is a tenaciously (persistent) adherent deposit that forms on tooth surface. It consists of an organic matrix containing a dense concentration of bacteria. 3. The bacterial activity in the plaque: Plaque contains bacteria that are acid producing. Mutans streptococci are believed to be the most important bacteria in the initiation and progress of dental caries. 4. Substrates: Bacteria utilize fermentable carbohydrates for energy and the end products of the glycolytic pathway in bacterial metabolism are acids. Sucrose is the fermentable carbohydrate most frequently implicated but bacteria can use all fermentable carbohydrates, including cooked starches. Interaction of these factors can be illustrated as follows- Bacteria + Sucrose = Acid + Susceptible tooth surface = Carries Plaque Ways of preventing dental carries: A. Increasing the resistance of the tooth surface enamel against acid products: The resistance of the tooth surface enamel to acid attack can be very greatly enhanced by the incorporation of minute amounts of Fluoride ion so that the hydroxyapatite crystals become fluoroapatite (which are solubility resistant against acid). The principal mode of action of all fluorides (tooth pastes, rinses, gels and community water fluoridation) is its topical effect on enamel. B. Diet modification: Minimizing intake of dietary refined carbohydrate and good dental hygiene prevent growth of bacteria that contribute to the development of caries. For this purpose sweets and other dietary refined sugar should be limited to mealtimes. Frequency of intake is more important than overall quantity as every time we eat there is a chance of plaque formation. ‘Snacking’ between meals should be avoided. The frequent consumption of soft drinks is another major problem, these being not only cariogenic but extremely erosive (since they are acidic). C. Plaque removal: Proper brushing of the teeth is effective in preventing and removing dental plaque in all areas except those between the teeth and deep fissures. Ideally, tooth brushing should be carried out twice a day and emphasis should be placed on brushing just before bed. Use of dental floss or tape removes plaque from between adjacent tooth surfaces; deep pits and fissures may be sealed by the application of resins. The sealant may need to be replaced periodically.
- 7. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 7 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU Parents should be advised to begin cleaning their children’s teeth from when those first erupt. Gauze or a cloth on a finger, or a small very soft toothbrush may be used to remove the plaque. D. Early detection and dental restorations: It offers the best form of control once caries has formed. Fluorides in preventing dental carries: Mechanism of action: The principal mode of action of all fluorides (tooth pastes, rinses, gels and community water fluoridation) is its effect on enamel. The resistance of the tooth surface enamel to acid attack can be very greatly enhanced by the incorporation of minute amounts of fluoride ion so that the hydroxyapatite crystals become fluoroapatite. The formation of this solubility resistant form explains the mode of action of fluorides as preventive agents. In the child, the developing tooth will receive its necessary building materials from the blood plasma and thus the enamel fluorine content at this Point will be completely dependent on systemically absorbed fluorine. After tooth eruption, maturation of the enamel takes place and a great deal of fluoride uptake is from topical route. Hence it may be assumed that fluoride acts in two complementary ways- by systemic action and by its topical action. Fluoride therapy (Systemic fluoridation and topical fluoridation): Fluoride therapy for the prevention of dental caries is considered under the two main headings: 1. Systemic fluoridation 2. Topical fluoridation. Systemic fluoridation: This can be done in following ways- i. Fluoridation of public water supplies: An optimal level of fluoride in the water supply provides significant protection against caries. The optimal concentration depends on the annual average temperature of the community as temperature influences the amount of daily water intake. Temperature ranging between 14.7o to 17.7°C, the optimal level of fluoride is 1 part per million (ppm). The adjustment of the fluoride concentration of public water supplies to 1 ppm is necessary in low-fluoride areas. Most commonly, fluoride is added in the form of hexafluorosilicic acid or sodium hexafluorosilicate, but sodium silicofluoride and sodium fluoride have also been used. In a warmer climate slightly less than 1 ppm is sufficient. The effect of fluoride in drinking water persists in between 8 to 18 years of age i.e. during tooth formation and mineralization. ii. Fluoride supplement: Fluoride supplements like tablet, drops, lozenges, table salt etc. offer an alternative source of systemic fluoridation where water fluoridation is not feasible.
- 8. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 8 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU These supplements are usually administered continuously on a daily basis from birth to the pre-eruptive maturation of permanent teeth. If fluoride tablets are prescribed they should be chewed rather than swallowed whole. This will increase the topical benefit of fluoride. Topical fluoridation: A lifetime protection against dental caries results from the continuous use of low concentration fluoride. In addition to their use in caries prevention, topical fluorides may be used to control established caries lesions. This is effective for both adults and children. Such topical products are i. Fluoride tooth paste: The use of fluoride toothpastes has led to a 25% reduction in the prevalence of caries in industrialized countries. On the other hand conventional tooth pastes contain approximately 1 mg F/g paste (1000-1100 ppm of fluoride) added as sodium fluoride such as sodium monofluorophosphate (SMFP) or stannous fluoride. ii. Fluoride mouth rinses: Studies showed that supervised fluoride-rinse programs reduce caries by 20-50%. Weekly 0.2% NaF and daily 0.05% NaF rinses were considered to be ideal public health measures. iii. Fluoride varnishes: Fluoride varnishes were developed to prolong contact times between fluoride and enamel with a view to increasing the formation of fluoroapatite. Although fluoride varnishes firmly bind fluoride in enamel more than other topical fluoride preparations, the reduction of caries has been of the same order (approximately 30%). Example of a varnish product is Duraphat. It is an alcoholic solution of natural varnishes containing 50mg NaF/mL. This varnish remains on the teeth for up to 12 hours and there is still fixation of fluoride evident up to 48 hours after application. iv. Concentrated fluoride gels and solutions (APF gels): Acidulated phosphate fluoride (APF) gels, containing 1.23% fluoride are used for professional applications and consist of a mixture of NaF, HF and orthophosphoric acid. The incorporation of a water-soluble polymer (i.e. sodium carboxymethyl cellulose) into aqueous APF produces a viscous solution that improves the ease of application. APF gels are mainly used for the prevention of caries development. Side effects of fluoride therapy - dental fluorosis: Dental fluorosis is chronic fluorine poisoning, sometimes marked by mottling of tooth enamel. It may result from excessive exposure to fluorides from a wide variety of dietary, water-borne, and supplemental sources. There is evidence to show that mild fluorosis will occur with ingestion of 2 mg or more of fluoride per day.
- 9. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 9 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU Sodium fluoride (NaF): NaF occurs as a white odorless powder which is soluble in water and insoluble in alcohol. It is prepared by interaction of 40% HF with an equivalent quantity of NaOH or Na2CO3. OHNaFNaOHHF 2 Stannous fluoride (SnF2): SnF2 occurs as a white, crystalline powder and has a bitter, salty taste. It is freely soluble in water and practically insoluble in alcohol, ether and chloroform. It is prepared by dissolving stannous oxide in 40% HF and the solution is evaporated out of contact with air. OHSnFHF2SnO 22 Use of NaF and SnF2: They are used as dental carries prophylactic. When teeth are being formed, ingestion of these fluorides is effective. Afterwards they are effective only topically. The application result in the corporation of fluorine to hydroxyapatite and flouroapatite is formed. Thus it makes changes in the outer layer of the enamel and exposed dentin making them more resistant to acidic erosion and dental carries. Dentifrices: Definition: Dentifrices are the substances that are used with a toothbrush for the removal of bacterial plaque, food debris, stains and calculus (mineralized dental plaque) only from the accessible surfaces of the tooth. Ideal properties of dentifrices: i. It should not be harmful to the oral tissues and fluid. ii. If it is ingested it shouldn’t be harmful to the GIT. iii. It should not stain teeth. iv. It should not be scratching to the enamel surface of tooth. v. It should have pleasant odor and taste. Types of dentifrices: Commercial dentifrices are generally available in two forms- i. Powder ii. Paste and gels Ingredients of dentifrices: Ingredients of powder form of dentifrices are i. Abrasives ii. Foaming agents iii. Flavoring agents Ingredients of paste dentifrices are i. Abrasives ii. Foaming agents iii. Humectants
- 10. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 10 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU iv. Binders and thickening agents v. Preservatives vi. Therapeutic agents vii. Flavoring agents viii. Sweetening agents ix. Coloring agents x. Water Functions of these components are given below: Abrasives: Abrasives are solid cleansing materials. It gives mechanical aid to the dentifrice and helps to remove debris and stains from the tooth surface. These agents must be nontoxic and should provide a maximum cleansing action with a minimum of abrasion to the dental hard tissue. In general dentifrice abrasives are inorganic salts that are relatively insoluble. CaCO3 is widely used as abrasive. Other abrasives used are – insoluble Na-metaphosphate (Na3PO3), anhydrous and hydrous Ca-monohydrogen phosphate (CaHPO4 and CaHPO4.2H2O), Ca- pyrophosphate (Ca2P2O7), MgCO3, hydrated Al2O3, silicates or dehydrated silica gels etc. Foaming agents: Foaming agents flushes and cleanse the cavity. Consumers prefer to use foaming dentifrices. Synthetic detergents which not only blend well with other constituents of the preparation but also provide optimal foaming capabilities are incorporated in the dentifrice formulations. Sodium lauryl sulfate is the most commonly used foaming agent. Other foaming agents shown to be useful include sodium N-lauryl sarcosinate and sodium coconut monoglyceride sulfonate. Humectants: Humectants are incorporated into tooth paste formulations to prevent loss of water and subsequent hardening of the preparation upon exposure to air. The most frequently employed agents include sorbitol, glycerol and propylene glycol. Binders: Binders or thickening agents are used to stabilize dentifrice formulations to prevent separation of the liquid and solid phases, specially upon storage. Binders increase the viscosity of the preparation and help to keep it on the brush. The most widely used thickening agents are the natural gums such as gum tragacanth, gum karaya, sodium algenate and synthetic celluloses such as Na-CMC and methylcellulose. Preservatives: Preservatives are used to inhibit bacterial proliferation in the preparation. Aqueous solutions of humectants support the growth of bacteria and molds. Thus preservatives like benzoic acid or esters of p-hydroxybenzoic acid are commonly added to the preparation. Therapeutic agents:
- 11. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 11 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU The majority of dentifrices contain therapeutic agents such as fluoride slats. Fluoride salts inhibit carries. Common fluoride salts used in tooth paste are SMFP, MFP, stannous fluoride etc. Also antibacterial agent like triclosan (trichloro hydroxy diphenyl ether) is added. It is a white or off-white, crystalline powder. It has broad-spectrum antibacterial activity. Flavoring agents: For most people, the flavor is extremely important factor in choosing a dentifrice. The flavoring agents used in dentifrices must be compatible with other dentifrice constituents and yet provide a smooth pleasant flavor both during brushing and as an aftertaste. Flavor ingredients include spearmint, peppermint, wintergreen or cinnamon mint which are blended with other essential oils to produce a distinctive flavor. Sweetening agents: Sweeteners are added to almost all flavors. The most frequently added sweetener is saccharin. Coloring agents: To improve attractiveness and to help differentiate between products colors are added. Red, green, blue or chocolate colors are frequently used. Composition of ‘crest’ toothpaste: Stannous fluoride 0.4% Stannous pyrophosphate 1% Calcium pyrophosphate 39% Glycerin 10% Sorbitol (70% solution) 20% Water 29.6% Miscellaneous formulating agents Composition of Colgate toothpaste: Sodium monofluorophosphate (SMFP) 0.76% Insoluble sodium metaphosphate 41.85% Anhydrous dicalcium phosphate 5% Sorbitol 11.9% Glycerin 9.9% Sodium N-lauryl sarcosinate 2% Water 24.4% Miscellaneous formulating agents 4.2% Mouthwash: Halitosis (bad breath): Halitosis is the condition in which the breath smells unpleasant. Cause:
- 12. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 12 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU The most common cause of halitosis is the accumulation of plaque and food debris on oral hard and soft tissues. Bacteria in these plaques produce odoriferous sulfate substances which contribute significantly to bad breath. It may also be caused by a number of other local or systemic factors. Treatment: Until the causative factor is removed commercial mouth wash is recommended. It flushes away loose debris, mask bad breathe and provide a pleasant taste. Its effect last about 15-30 minutes.
- 13. Dental Products Arranged By- Md. Imran Nur Manik; B.Pharm.; M.Pharm.;(Thesis) RU Page 13 Prepared By- Shadid Uz Zaman At Tadir; B.Pharm.;M.Pharm; (Professional) DU Mouthwashes: Definition: Mouthwashes are medicated solutions used to cleanse or treat diseases of the oral mucosa, reduce halitosis and/or add fluoride to the teeth for control or prevention of dental carries. Uses: i. They are used in postoperative treatment and during the course of certain operative procedures to improve oral hygiene. ii. Some mouthwashes have anesthetic effect on the oral mucosa and used to relief pain associated with denture sore spots, herpetic infections and aphthous ulcers (lesion in mucous membrane, in this topic; of oral mucosa). iii. Patients with painful lesions in the mouth administered before eating to give comfort. Examples: Materials that are used in mouth wash include i. Chlorhexidine ii. NaCl iii. Povidone-iodine iv. NaF v. Cetylpyridinium chloride vi. Na borate vii. H2O2 Listerine (thymol and other essential oils), cepacol (cetylpyridinium chloride) and scope (cetylpyridinium chloride and domiphen bromide) are some of the popular mouthwashes which are effective against halitosis.
