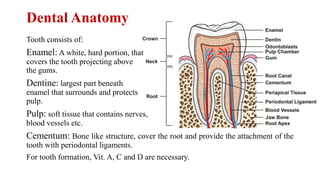
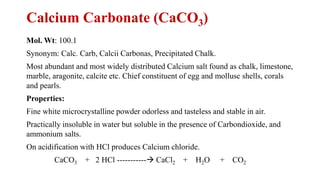
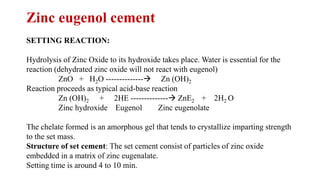
The document discusses dental hygiene, the common dental problems in India, and offers detailed explanations of dental anatomy, common terms, and treatments. It emphasizes the importance of education and regular dental care to prevent issues such as dental caries and periodontitis, as well as the roles of various substances like fluoride, zinc chloride, and zinc eugenol cement in dental health. Additionally, it covers the chemical properties and uses of different compounds in dental products to maintain oral hygiene and treat dental conditions.