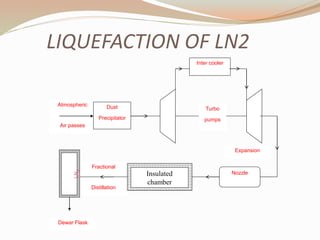
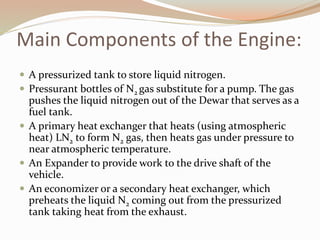
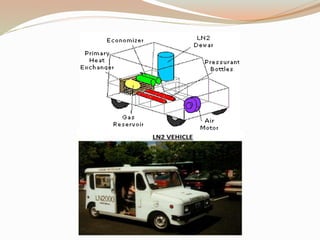
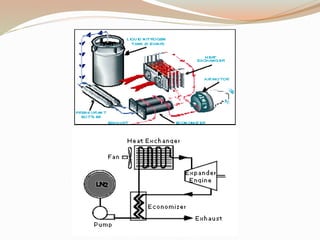
This document summarizes a proposed vehicle called the Cryocar that is powered by liquid nitrogen. The Cryocar works similarly to a steam engine by pressurizing and vaporizing liquid nitrogen using ambient heat, which expands to drive the vehicle. It has zero emissions and refueling takes only a few minutes. However, issues around safety from nitrogen leaks and the energy required to liquefy nitrogen have prevented commercialization. The document outlines the components, working principle, advantages over electric vehicles, drawbacks, and potential solutions of the Cryocar concept.















![REFERENCE
[1] “LN2000”, University of Washington Research Team, Sept. 18, 2007
<http://www.aa.washington.edu/AERP/CRYOCAR/CryoCar.htm>."
[2] “Liquid Nitrogen”, Wikipedia Online Encyclopedia, Sept. 13, 2007
<http://en.wikipedia.org/wiki/Liquid nitrogen>.
[3] “Cryogenic Chilling and Freezing”, BOC Gases, Sept. 18, 2007 <http://www.boc-gases.
com/products and services/by process/cryogenic_chilling_and_freezing.asp>.
[4] “Cryogenic Paint Removal”, Sig Attilio Bernasconi, Sept. 18, 2007
<http://www.p2pays org/ref/10/09444.htm](https://image.slidesharecdn.com/cryocar1-141214033602-conversion-gate01/85/Cryocar-1-21-320.jpg)
