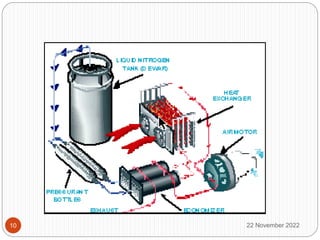
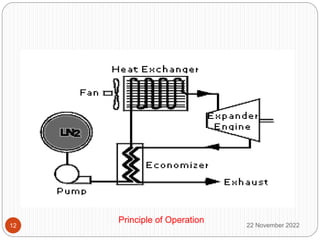
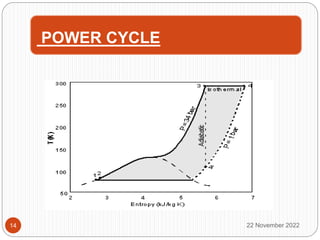
The document discusses cryogenics and cryogenic heat engines. It describes how a cryocar works using liquid nitrogen as fuel. Liquid nitrogen is pressurized and vaporized in a heat exchanger, producing pressurized nitrogen gas that drives the engine. While this eliminates vehicle emissions, liquefying nitrogen requires energy and the technology has limitations regarding speed, leakage hazards, and lack of refueling infrastructure. More research is still needed to address safety and commercialization.