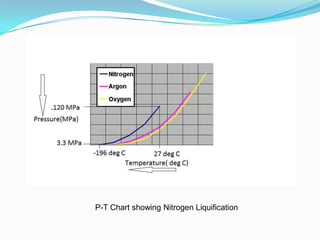
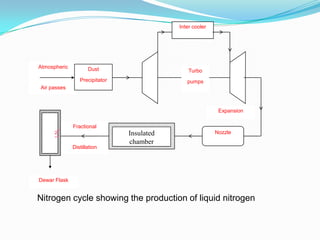
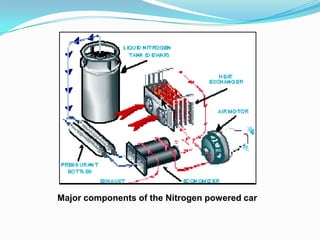
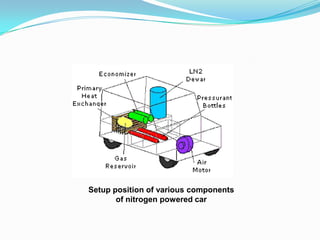
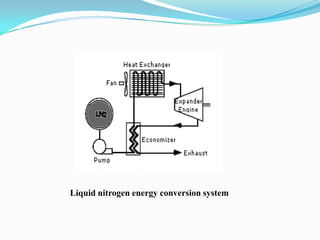
This document discusses a liquid nitrogen vehicle. It provides a history of liquid nitrogen vehicles being developed in 1997. The main components of the engine are described, including a pressurized liquid nitrogen tank, heat exchangers, and an expander. The principle of operation involves using ambient heat to vaporize the liquid nitrogen, which then drives the expander and vehicle. Advantages include zero emissions and a potential longer range than electric vehicles, while drawbacks include safety issues and energy required for liquefaction. More research is still needed before commercialization.