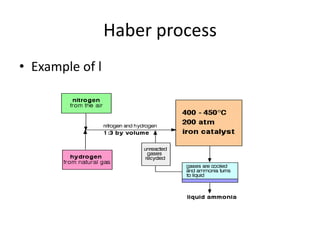
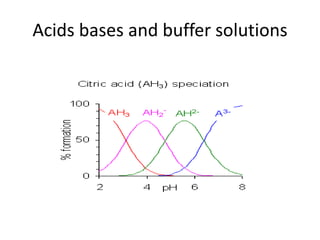
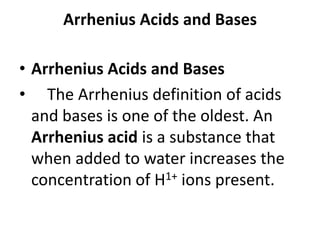
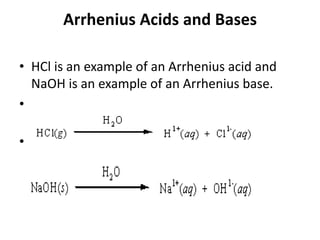
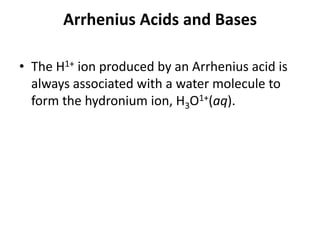
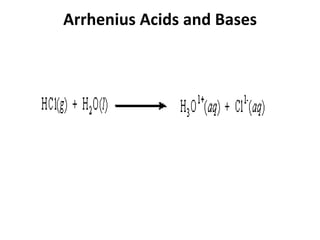
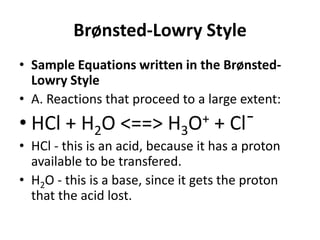
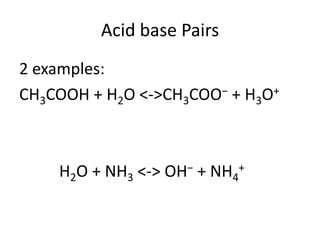
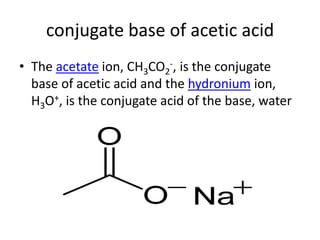
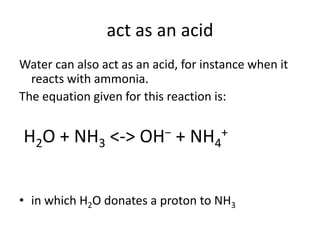
This document discusses Lewis acids and bases, strong acids and bases, pH and pOH scales, autoionization of water, and buffer solutions. It provides definitions and examples of Arrhenius acids and bases, Bronsted-Lowry acids and bases, and Lewis acids and bases. It also discusses properties of strong acids and bases, calculating pH and pOH, and how buffer solutions resist changes in pH through acid-base equilibriums.



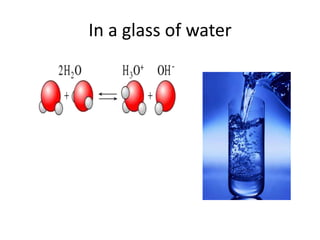
![Autoionization of Water
• Autoionization of Water
The equilibrium product Kw = [H+] [OH-]
is a constant at a definite temperature due to the
autoionization of water,
H2O = H+ + OH-.
• At 298 K, Kw = 10-14 and the following relationship
in any aqueous solution is obvious,
• ***pOH + pH = 14 at 298 K.](https://image.slidesharecdn.com/acidandbasechm141-thursday1goodday-121121141355-phpapp02/85/Acidandbase-chm141-thursday-1-goodday-6-320.jpg)
![Will need this soon
• ***pOH + pH = 14 at 298 K.
pH = -log[H+]](https://image.slidesharecdn.com/acidandbasechm141-thursday1goodday-121121141355-phpapp02/85/Acidandbase-chm141-thursday-1-goodday-7-320.jpg)
![We say
• The pH scale is defined as the negative log of
the concentration of H+: pH = -log[H+]
• The pOH scale is defined as the negative log of
the concentration of OH-, [OH-]:
• pOH = -log[OH-] With this scale, calculating
the pOH can be done in the same manner as
the pH scale.](https://image.slidesharecdn.com/acidandbasechm141-thursday1goodday-121121141355-phpapp02/85/Acidandbase-chm141-thursday-1-goodday-8-320.jpg)
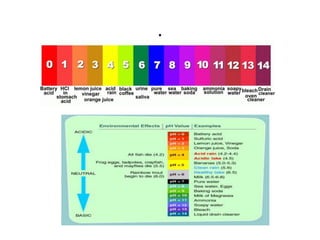












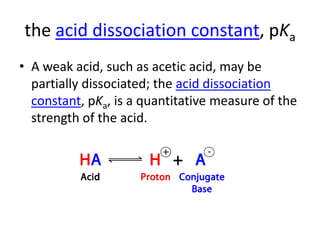












![concentrations of acids and bases
• The concentrations of acids and bases are
often expressed in terms of pH, and as an
educated person, you should have the skill to
convert concentrations into pH and pOH. The
pH is an indication of the hydrogen ion
concentration, [H+].](https://image.slidesharecdn.com/acidandbasechm141-thursday1goodday-121121141355-phpapp02/85/Acidandbase-chm141-thursday-1-goodday-48-320.jpg)
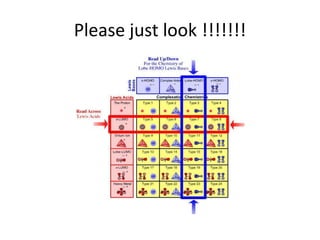
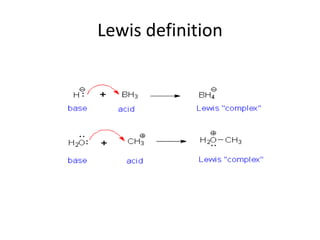
![The term Lewis acid
• The term Lewis acid refers to a definition of
acid published by Gilbert N. Lewis in 1923,
specifically: An acid substance is one which
can employ a lone pair from another molecule
in completing the stable group of one of its
own atoms.[1] Thus, H+ is a Lewis acid, since it
can accept a lone pair, completing its stable
form, which requires two electrons](https://image.slidesharecdn.com/acidandbasechm141-thursday1goodday-121121141355-phpapp02/85/Acidandbase-chm141-thursday-1-goodday-49-320.jpg)