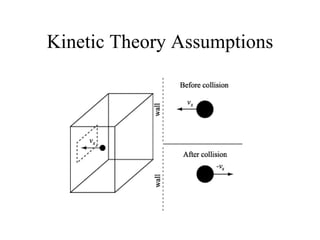
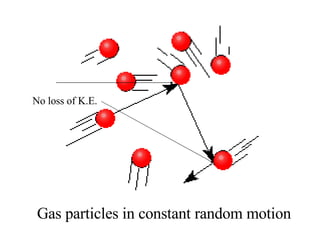
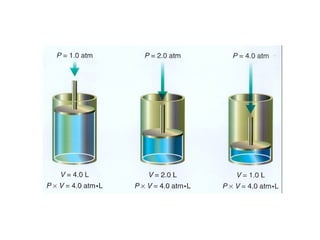
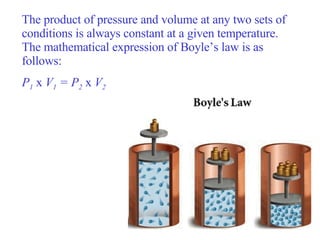
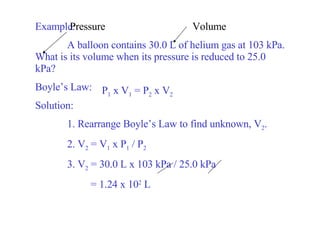
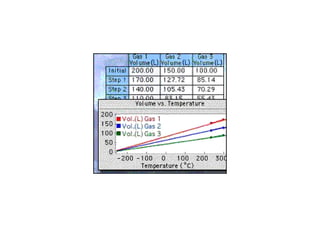
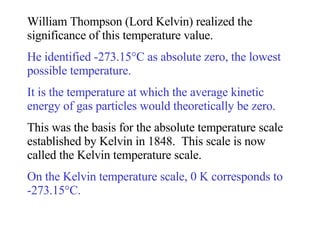
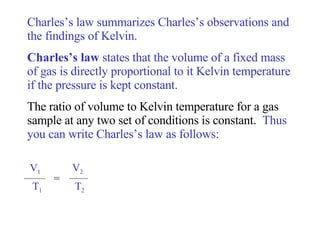
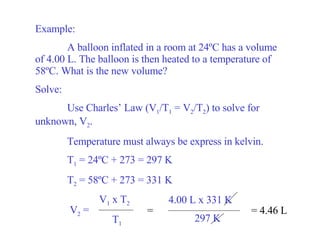
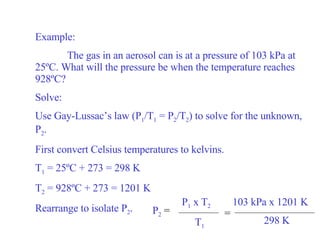
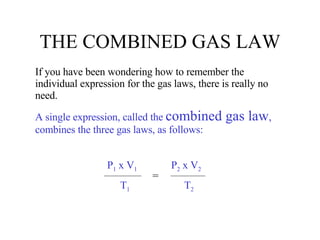
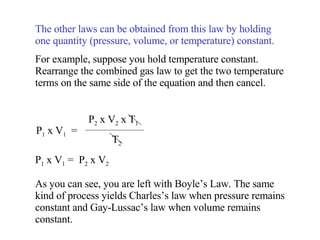
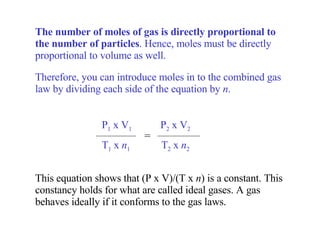
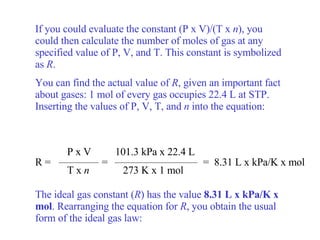
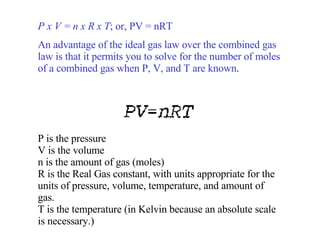
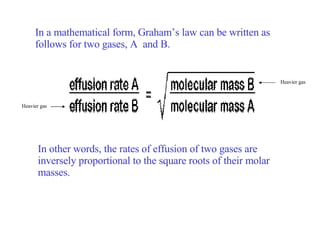
Kinetic molecular theory explains gas behavior using several assumptions: gas particles are small, far apart, and in constant random motion. The theory can predict how gas properties like pressure, volume, and temperature relate based on the kinetic energy and number of particles. Specifically, Boyle's law states that pressure and volume are inversely related at constant temperature, Charles's law relates volume and temperature directly at constant pressure, and Gay-Lussac's law directly relates pressure and temperature at constant volume. Together these combine to form the ideal gas law.