The document summarizes key periodic trends in the properties of elements as they relate to their position in the periodic table, including:
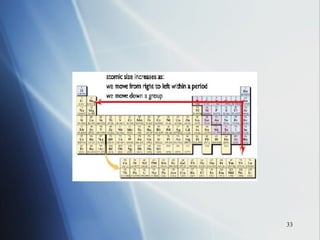
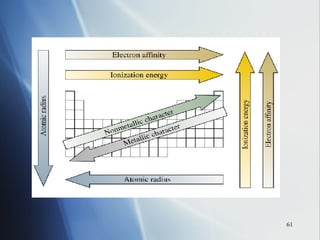
1) Atomic radius generally increases down a group and decreases across a period as more protons are added to the same principal energy level.
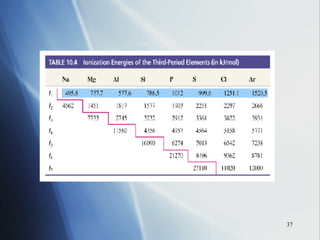
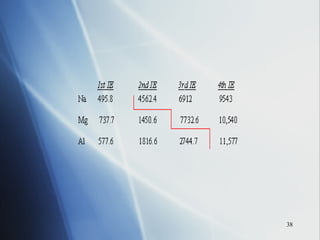
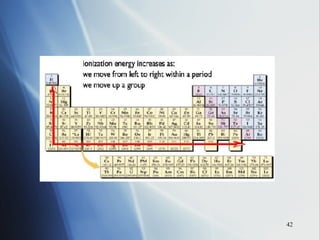
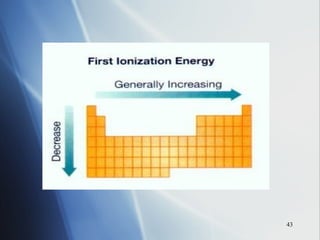
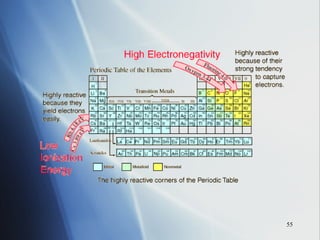
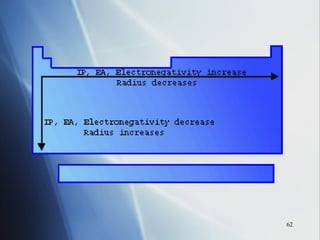
2) Ionization energy decreases down a group as atoms gain principal energy levels but increases across a period as more protons are added.
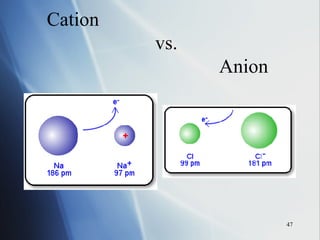
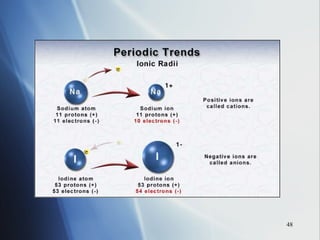
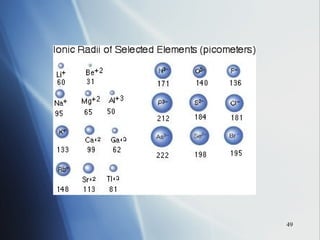
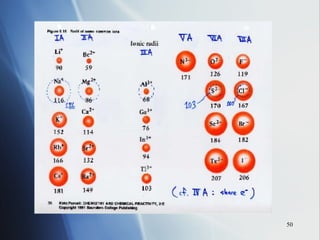
3) Positive ion size decreases and negative ion size increases relative to their parent atoms. Ion sizes also increase down a group.
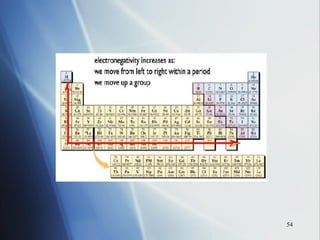
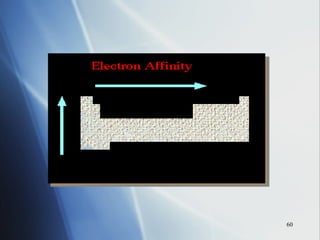
4) Electronegativity decreases down a group but increases across a period toward the nonmetals.








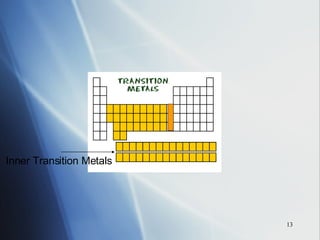







![4 . The inner transition metals . These are metallic elements in which the outermost s sublevel and nearby f sublevel generally contain electrons. The inner transition metals are characterized by the filling of f orbitals. Cerium [Xe]6 s 2 5 d 1 4 f 1 Thorium [Rn]7 s 2 6 d 1 5 f 1 If you consider both the electron configurations and the positions of the elements in the periodic table, another pattern emerges. The periodic table can be divided into sections, or blocks, that correspond to the sublevels that are filled with electrons. Notice the outermost “s” and “f” electrons are filling.](https://image.slidesharecdn.com/periodic-table-chapter-14-1193755948781507-2/85/Periodic-Table-Chapter-14-24-320.jpg)