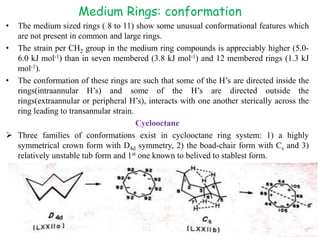
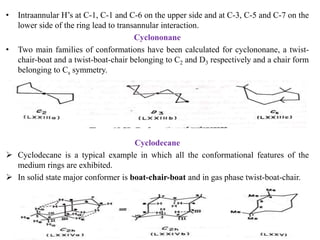
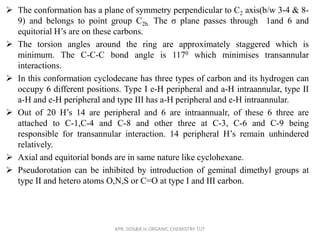
The document discusses the conformations of medium-sized carbocyclic rings from cycloheptane to cyclodecane. Cycloheptane exists in two sets of conformers, with the preferred conformers being twist-chair. Medium rings from cyclooctane to cyclodecane exhibit unusual features like intraannular and extraannular hydrogens leading to transannular strain. Cyclodecane preferentially adopts a boat-chair-boat conformation to minimize these interactions. Pseudorotation in these rings can be slowed by introducing substituents that restrict bond rotation.