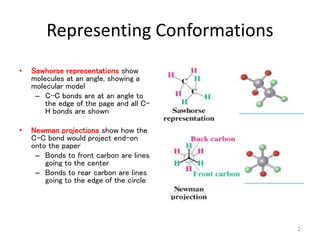
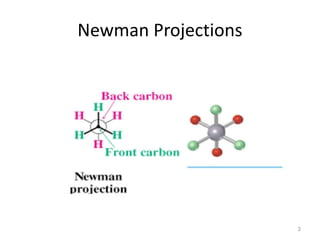
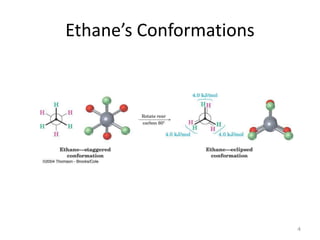
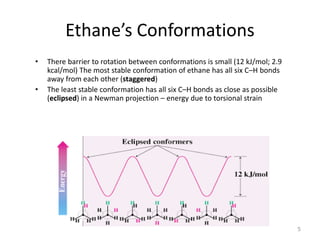
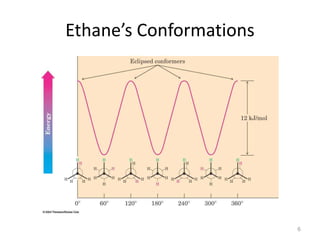
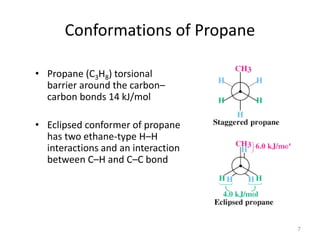
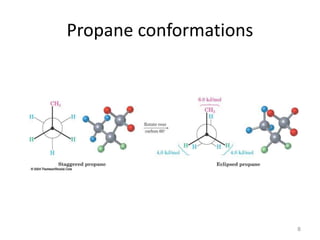
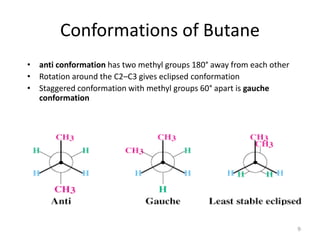
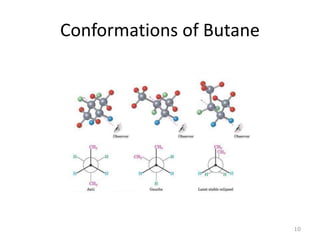
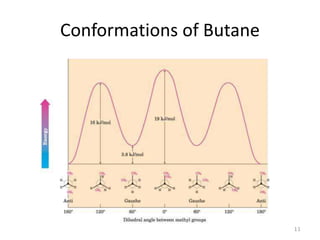
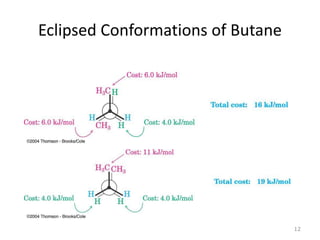
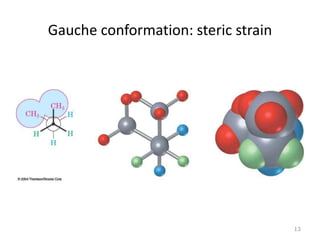
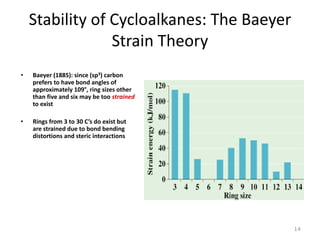
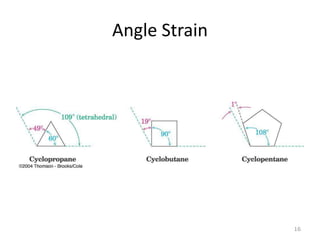
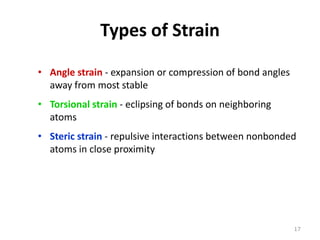
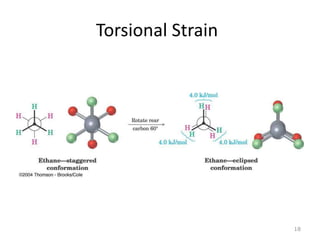
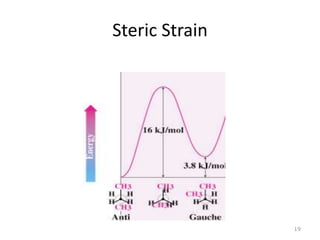
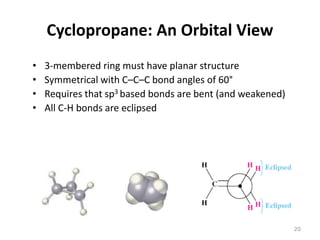
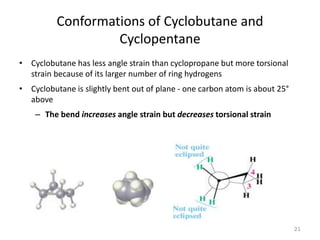
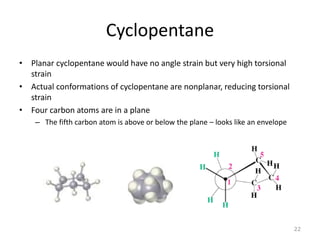
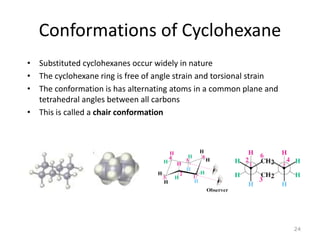
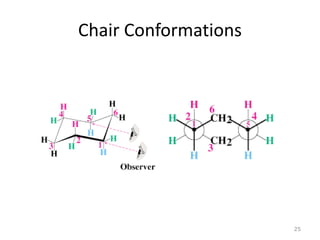
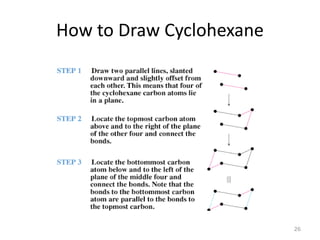
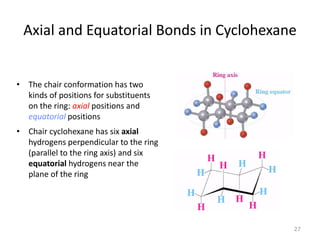
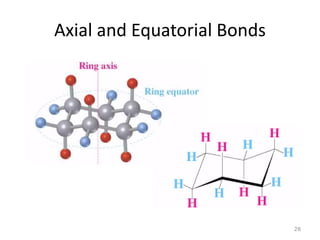
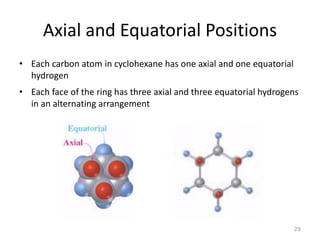
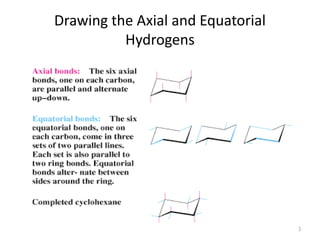
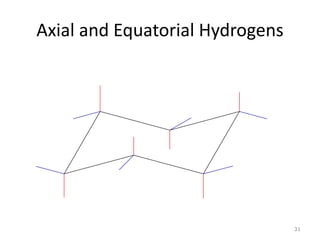
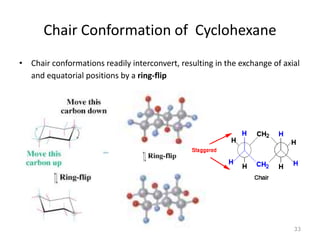
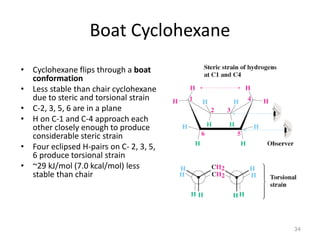
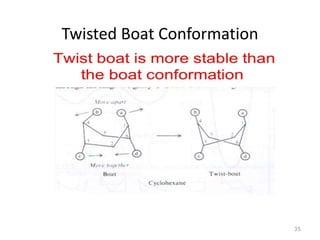
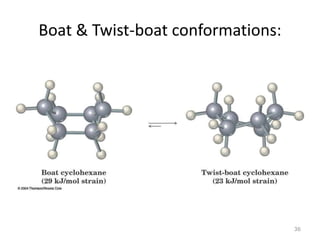
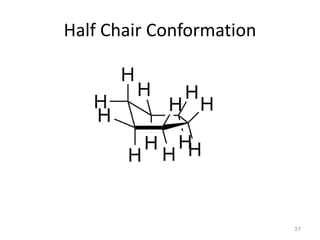
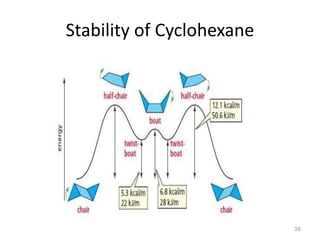
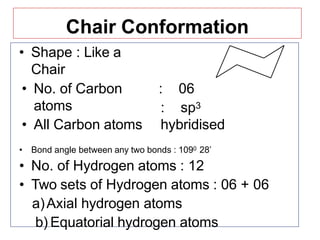
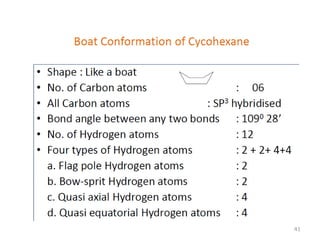
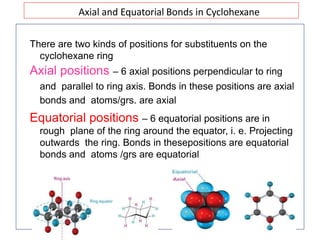
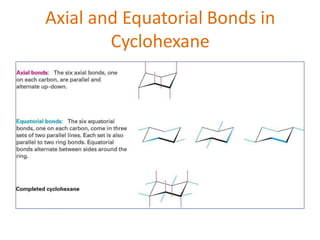
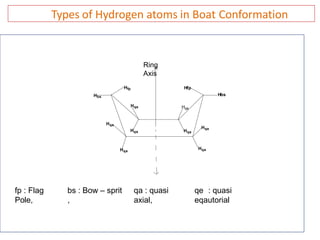
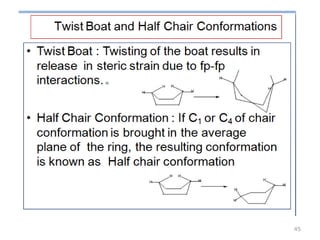
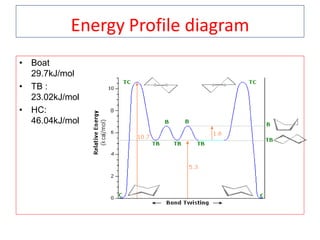
The document discusses conformational isomerism in small organic molecules like ethane, propane, butane, and cyclohexane. It explains how to represent molecular conformations using Newman projections and sawhorse diagrams. It describes the different conformations of these molecules, including staggered, eclipsed, anti, gauche, chair, boat, twist-boat, and half-chair. The preferred chair conformation of cyclohexane is explained in detail, with distinctions made between axial and equatorial positions and bonds. Energy barriers for interconversions between cyclohexane conformations are provided.