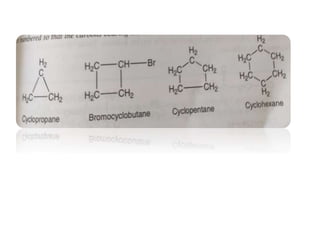
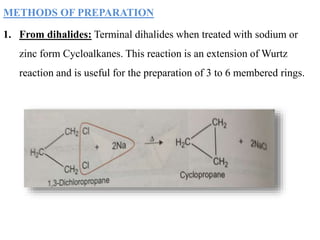
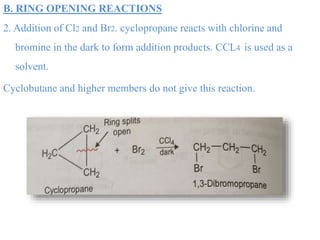
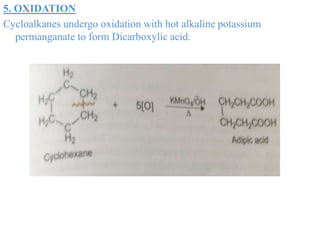
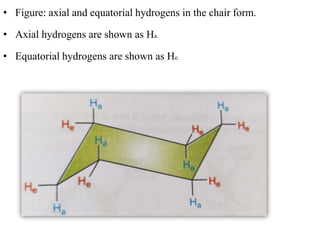
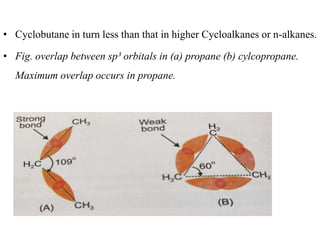
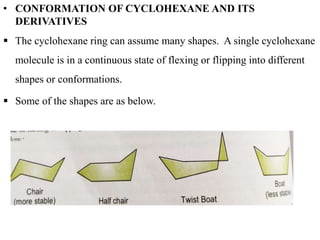
This document provides information about cycloalkanes. It discusses their structure, naming conventions, methods of preparation, physical and chemical properties, and theories explaining their stability. Cycloalkanes are saturated hydrocarbons that form rings, with the general formula CnH2n. The most important theories discussed are Baeyer's strain theory, which proposes bond angle deviations cause strain, and the Sachse-Mohr theory, which suggests higher cycloalkanes relieve strain by puckering out of planar structures. Cyclopropane is the least stable due to high bond angle deviations from tetrahedral.