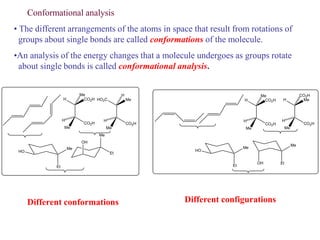
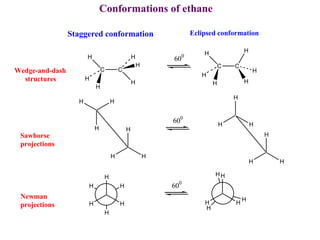
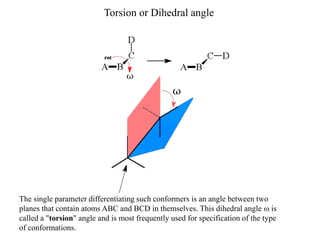
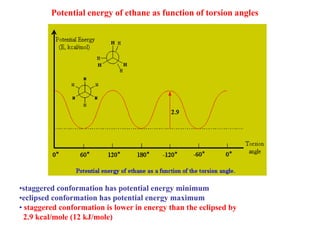
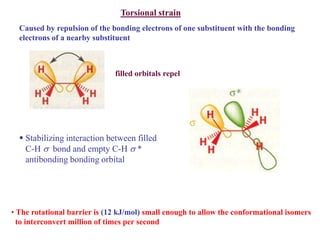
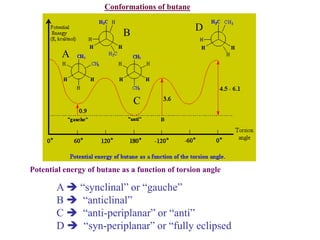
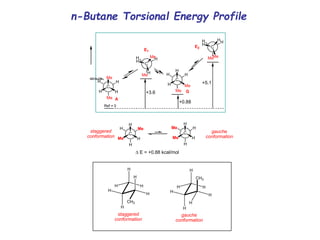
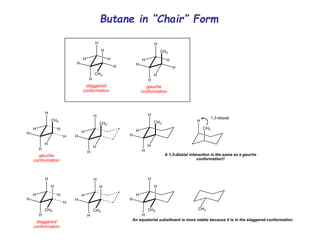
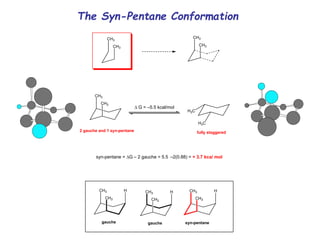
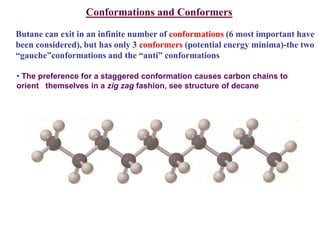
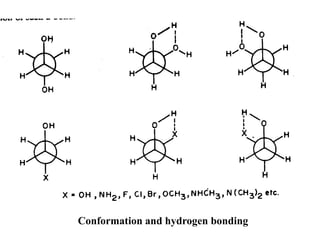
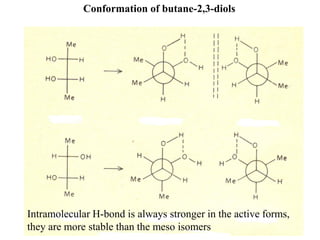
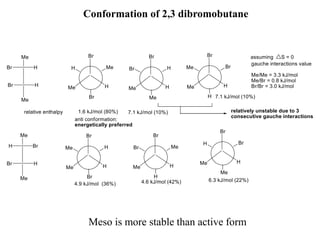
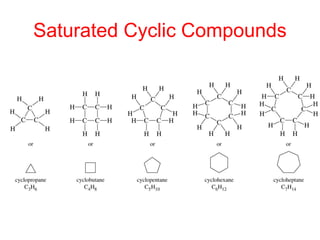
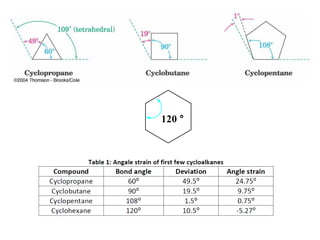
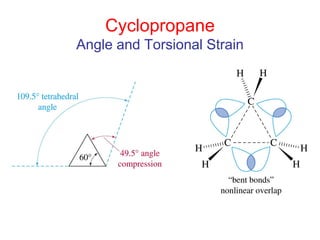
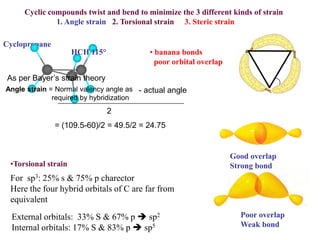
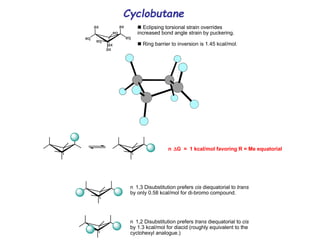
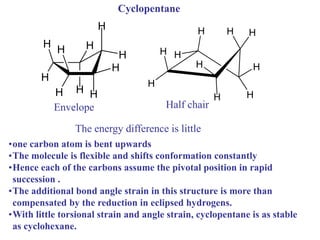
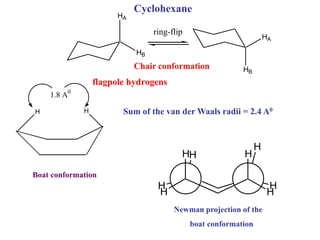
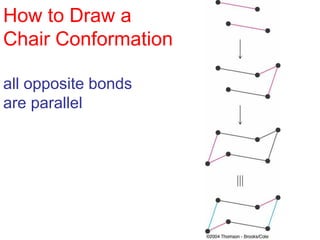
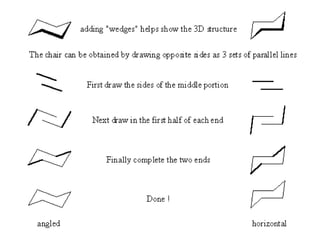
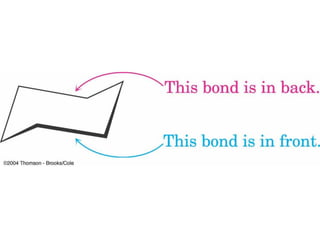
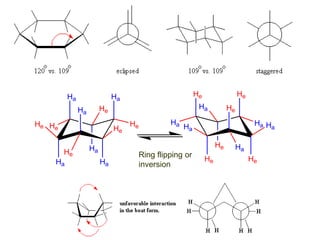
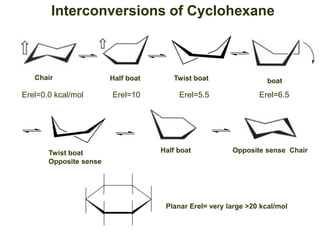
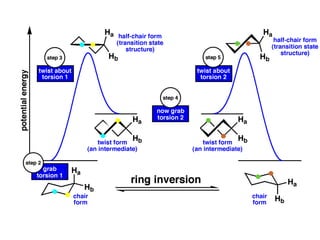
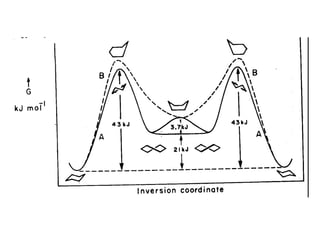
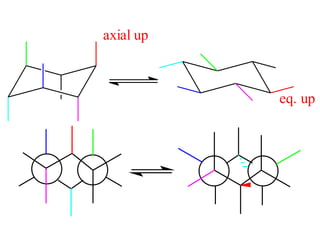
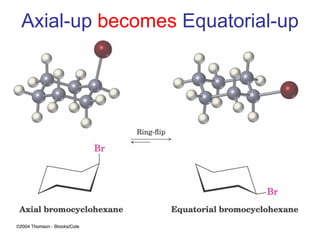
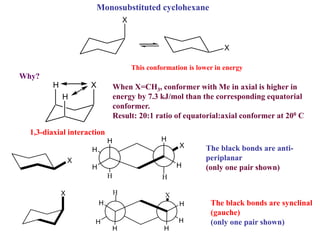
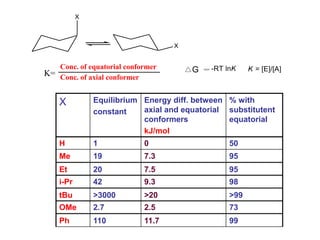
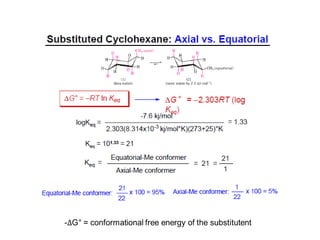
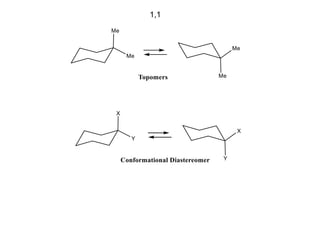
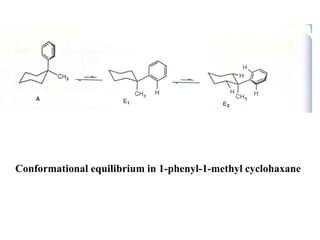
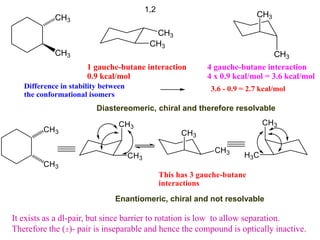
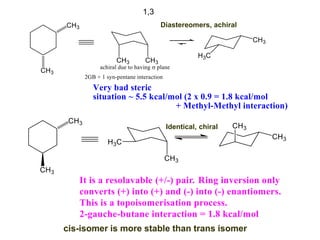
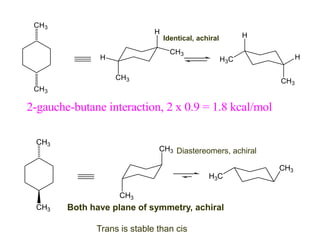
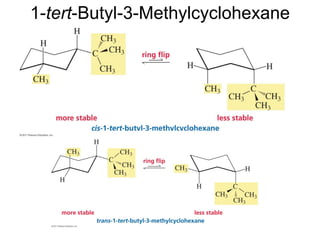
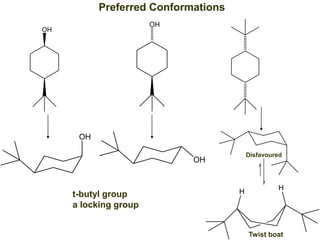
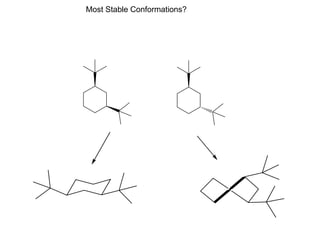
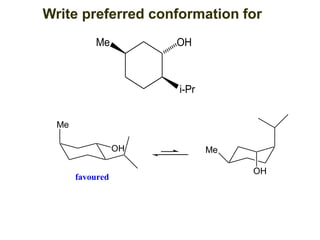
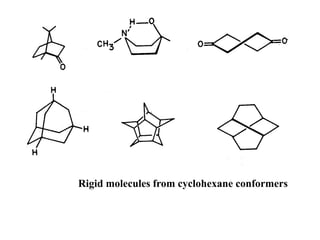
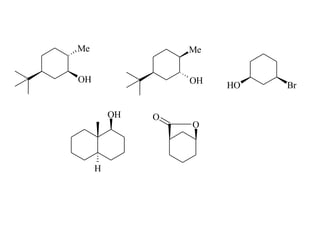
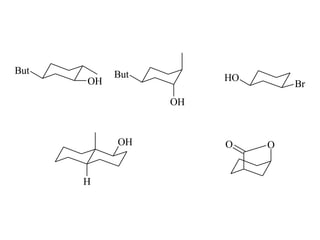
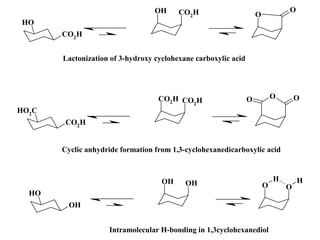
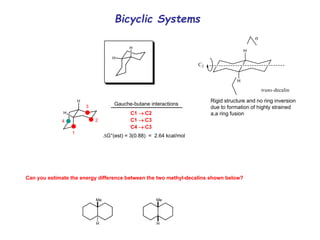
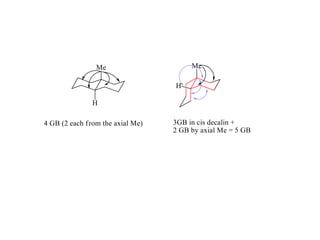
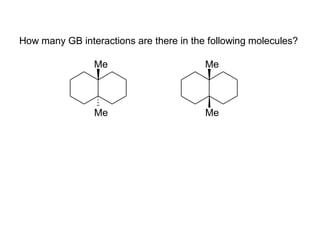
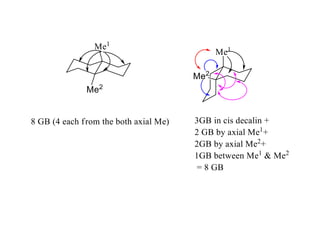
The document provides a comprehensive overview of conformational analysis, focusing on the different spatial arrangements of atoms resulting from rotations about single bonds in various molecules such as ethane and butane. It discusses concepts such as steric strain, torsional strain, and various conformations like staggered and eclipsed, emphasizing the energy differences between these arrangements. Additionally, it explains the stability of conformers in cyclic structures, including cyclohexane and cyclopentane, while outlining their conformational preferences and interactions.