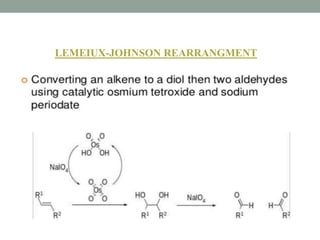
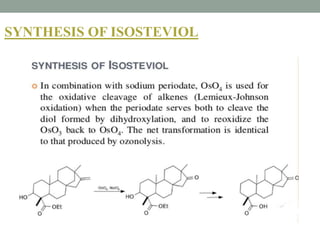
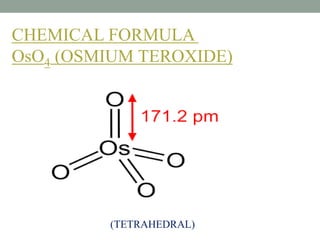
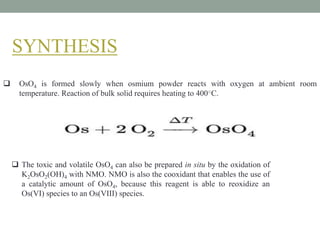
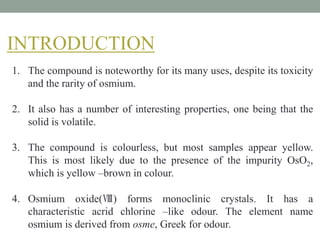
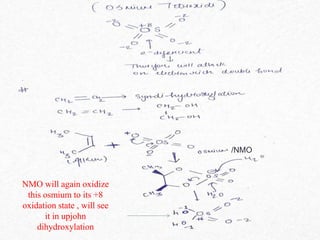
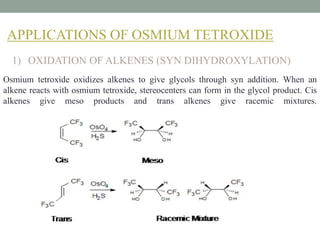
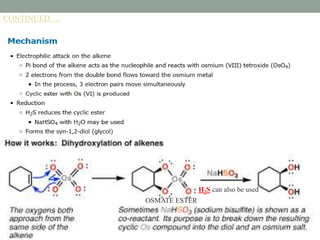
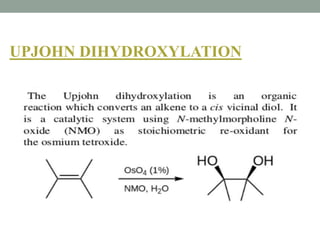
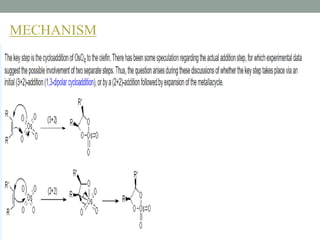
This document presents information about osmium tetroxide (OsO4). It discusses the chemical structure and synthesis of OsO4, noting that it forms slowly when osmium powder reacts with oxygen at room temperature. The document also covers the physical properties of OsO4, such as its solubility in organic solvents and water, melting point, and boiling point. Finally, the main application discussed is OsO4's use in the oxidation of alkenes to form glycols through syn addition, with examples given of the Upjohn dihydroxylation, Sharpless asymmetric dihydroxylation, and use of OsO4 in antitumor drug synthesis.




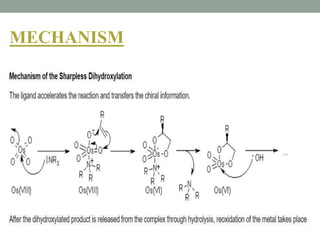
![The Sharpless Dihydroxylation or Bishydroxylation is used in the enantioselective
preparation of 1,2-diols from prochiral olefins. This procedure is performed with
an osmium catalyst and a stoichiometric oxidant [e.g. K3Fe(CN)6 or N-
methylmorpholine oxide (NMO)]; it is carried out in a buffered solution to ensure a
stable pH, since the reaction proceeds more rapidly under slightly basic conditions.
Enantioselectivity is achieved through the addition of enantiomerically-enriched
chiral ligands [(DHQD)2PHAL(β), (DHQ)2PHAL(α) or their derivatives]. These
reagents are also available as stable, prepackaged mixtures (AD-mix α and AD-mix
β, AD = asymmetric dihydroxylation) for either enantiopreference.
SHARPLESS ASYMMETRIC DIHROXYLATION](https://image.slidesharecdn.com/osmiumtetroxide-210603084552/85/Osmium-tetroxide-13-320.jpg)