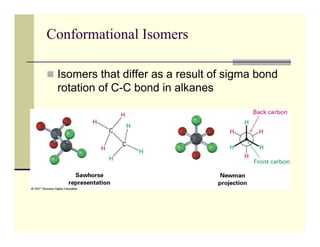
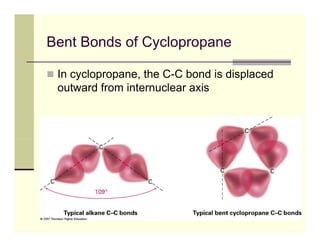
1) Conformational isomers differ due to rotation around sigma bonds, causing changes in molecular shape but not bond connectivity. Cycloalkanes exist as conformational isomers due to limitations on bond rotation in the ring structure.
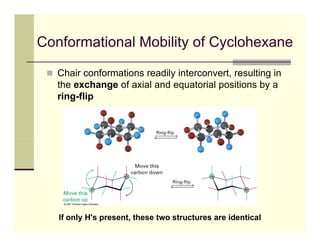
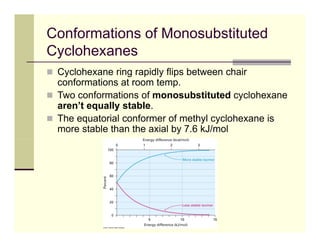
2) The chair conformation of cyclohexane minimizes torsional strain, with alternating carbons above and below the plane. Substituents can be in axial or equatorial positions, with equatorial being more stable due to less 1,3-diaxial interactions.
3) In trans-1,2-dimethylcyclohexane, the diequatorial conformer is more stable than the diaxial by 11.4 kJ/mol due to
















![Stability of Cycloalkanes: The Baeyer
St i Th
Strain Theory
Baeyer (1885): since
carbon prefers to have
bond angles of
approximately 109°,
i i th th
ring sizes other than
five and six may be too
strained to exist
Rings from 3 to 30 C’s
do exist but are
strained due to bond
bending distortions and
steric interactions [ H/CH2 (ring) - H/CH2 (acyclic) ] * n
(CH2)n + 3n/2 O2 n CO2 + n H2O + Heat](https://image.slidesharecdn.com/day4lecture-221110084222-97a9cb70/85/day-4-lecture-pdf-18-320.jpg)
















![Additional Cyclic Structures
H H
Additional Cyclic Structures
H
H
H
H
Boat Cyclohexane
H H
H H
Fused Polycycles
H
H
H H
trans-decalin cis-decalin
Bridged Polycycles
bicyclo[2.2.1]heptane](https://image.slidesharecdn.com/day4lecture-221110084222-97a9cb70/85/day-4-lecture-pdf-38-320.jpg)