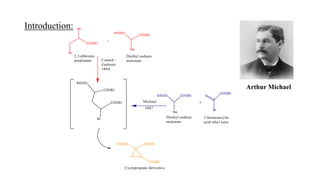
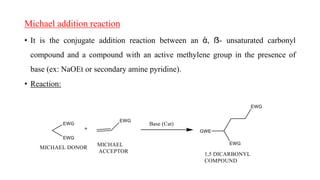
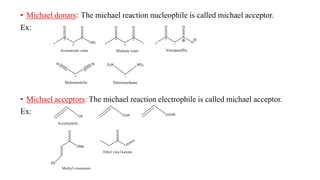
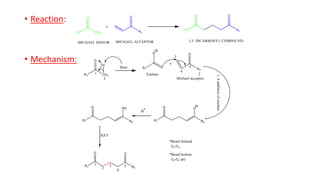
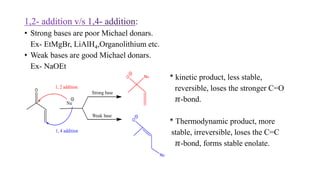
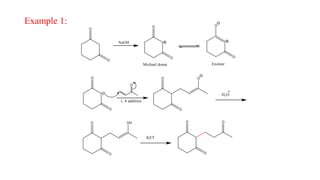
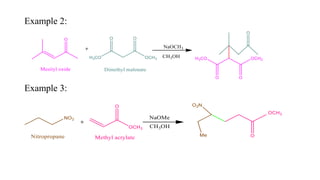
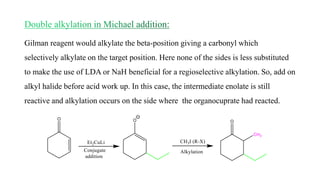
Michael addition reaction involves the conjugate addition of a nucleophile to an α,β-unsaturated carbonyl compound. It proceeds through a reversible 1,2-addition of the nucleophile to the β-carbon, which can then undergo irreversible proton elimination to form the more stable thermodynamic enolate product by removing the π-bond of the C=C. This reaction is useful for forming C-C bonds and natural products, with examples given of its application in multi-step syntheses using further carbonyl reactions like aldol condensation.