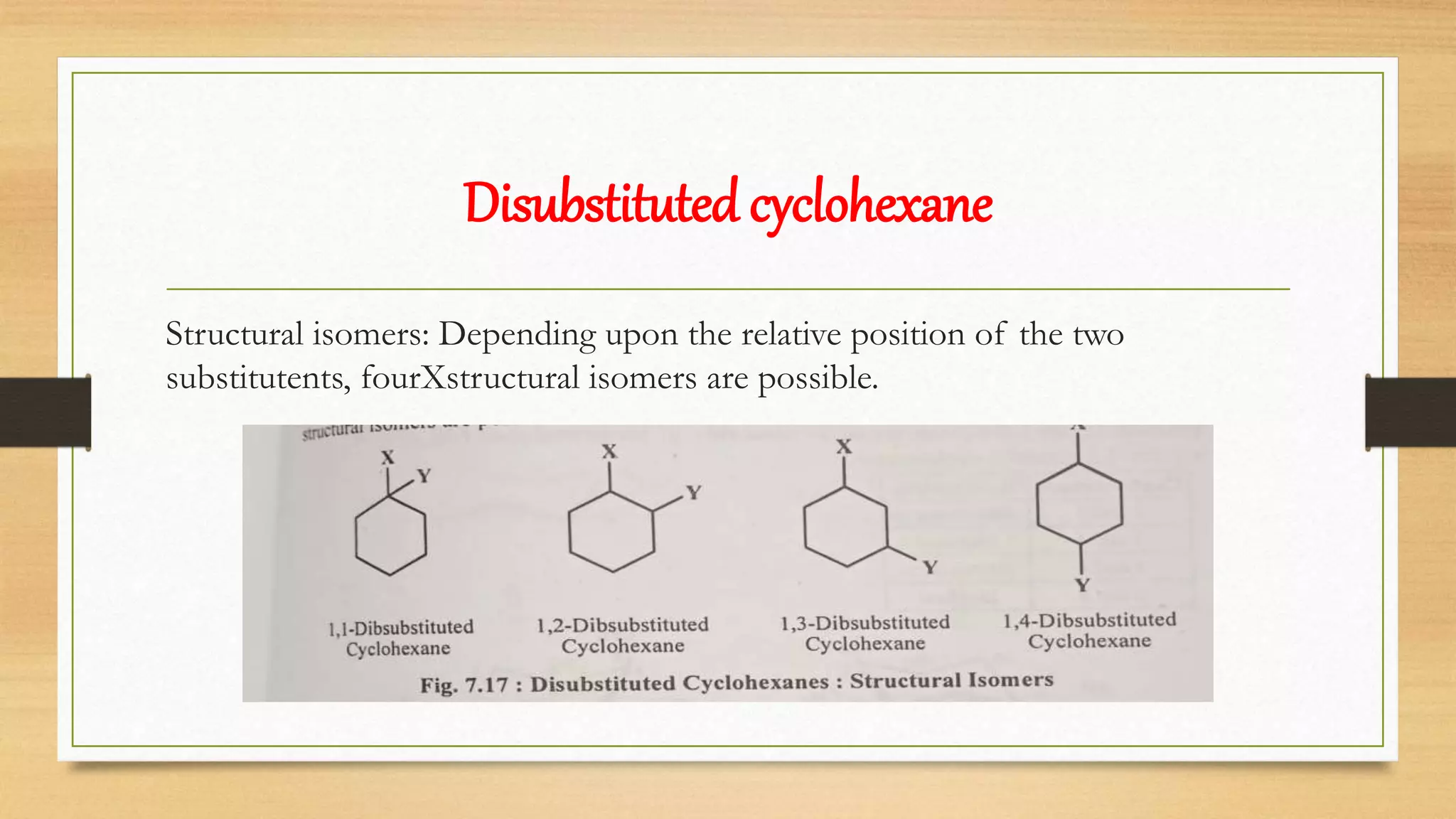
Cyclohexane exists in stable chair and boat conformations. The chair conformation is more stable due to minimal angle and torsional strain. Substituents on cyclohexane can exist in axial or equatorial positions, with the equatorial position generally being more stable due to less 1,3-diaxial interactions. Disubstituted cyclohexanes can exist as cis or trans isomers, with the trans isomer being more stable for 1,2-disubstituted cyclohexanes due to less van der Waals strain.