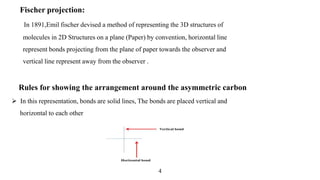
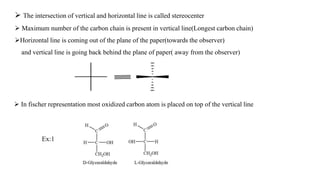
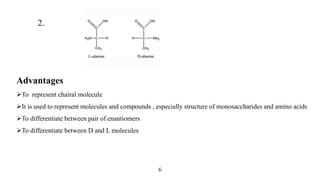
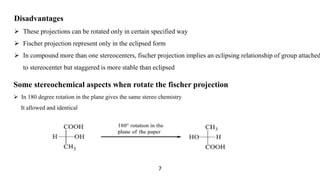
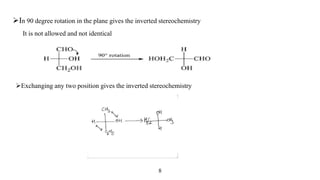
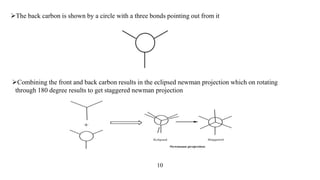
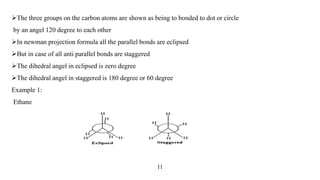
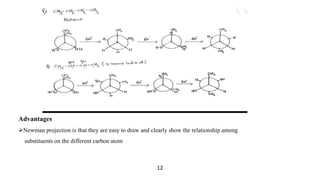
This seminar presentation discusses Fischer and Newman projections, two methods for representing the 3D structures of molecules in 2D. It outlines the definitions, advantages, and disadvantages of each projection, along with key stereochemical aspects and rules for proper representation. The conclusion emphasizes the utility of these projections in illustrating chirality and chemical conformations.