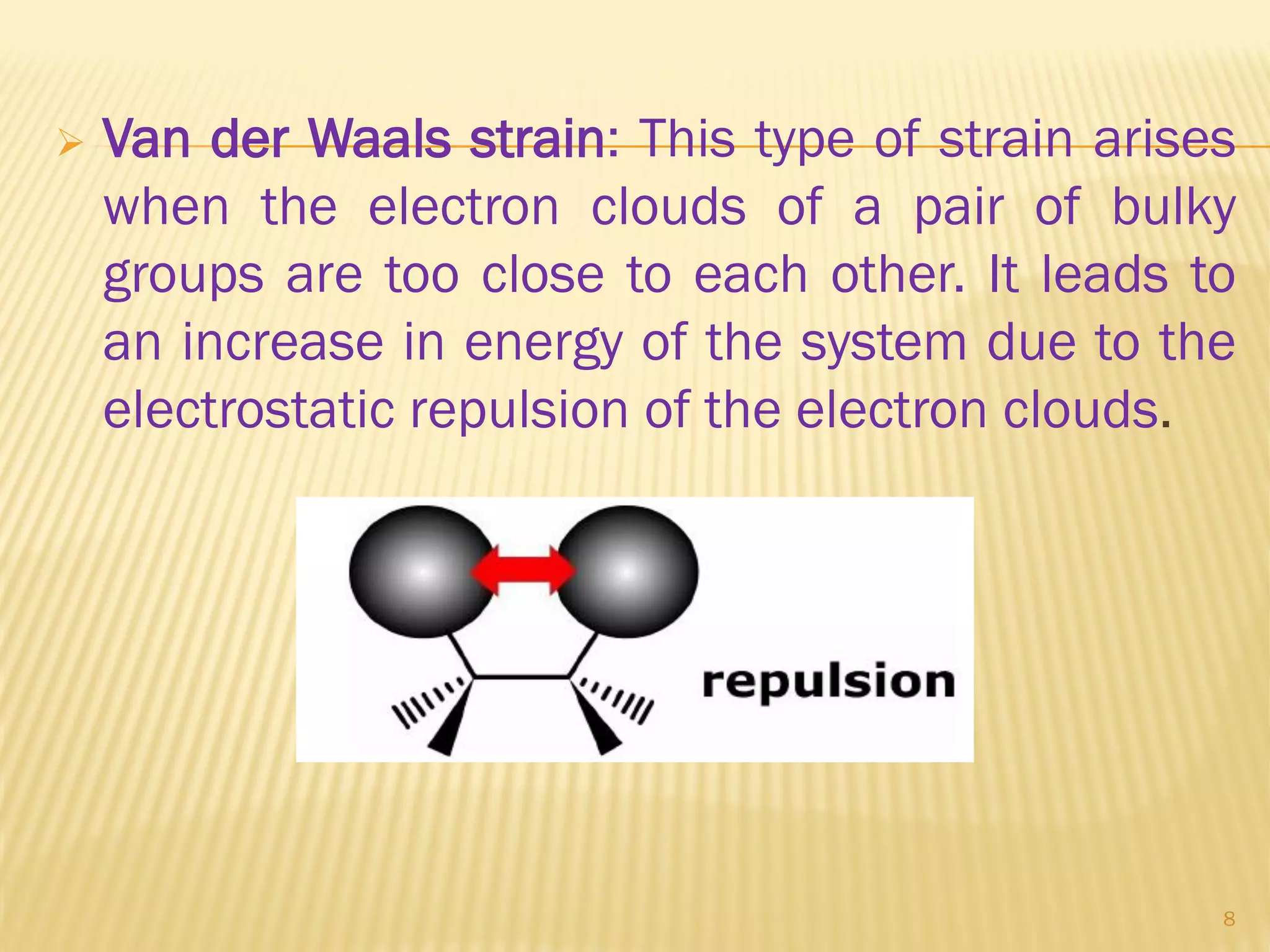
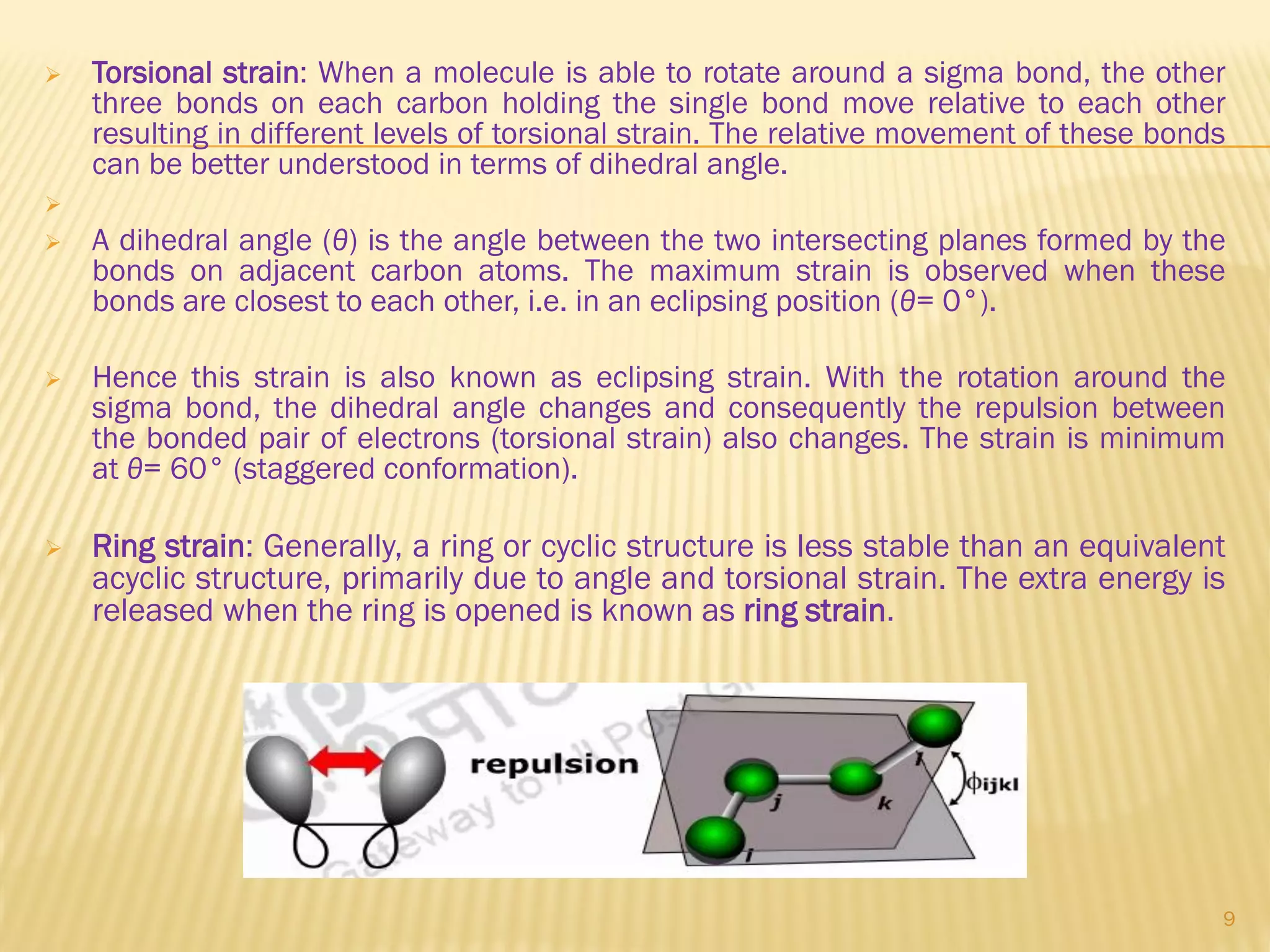
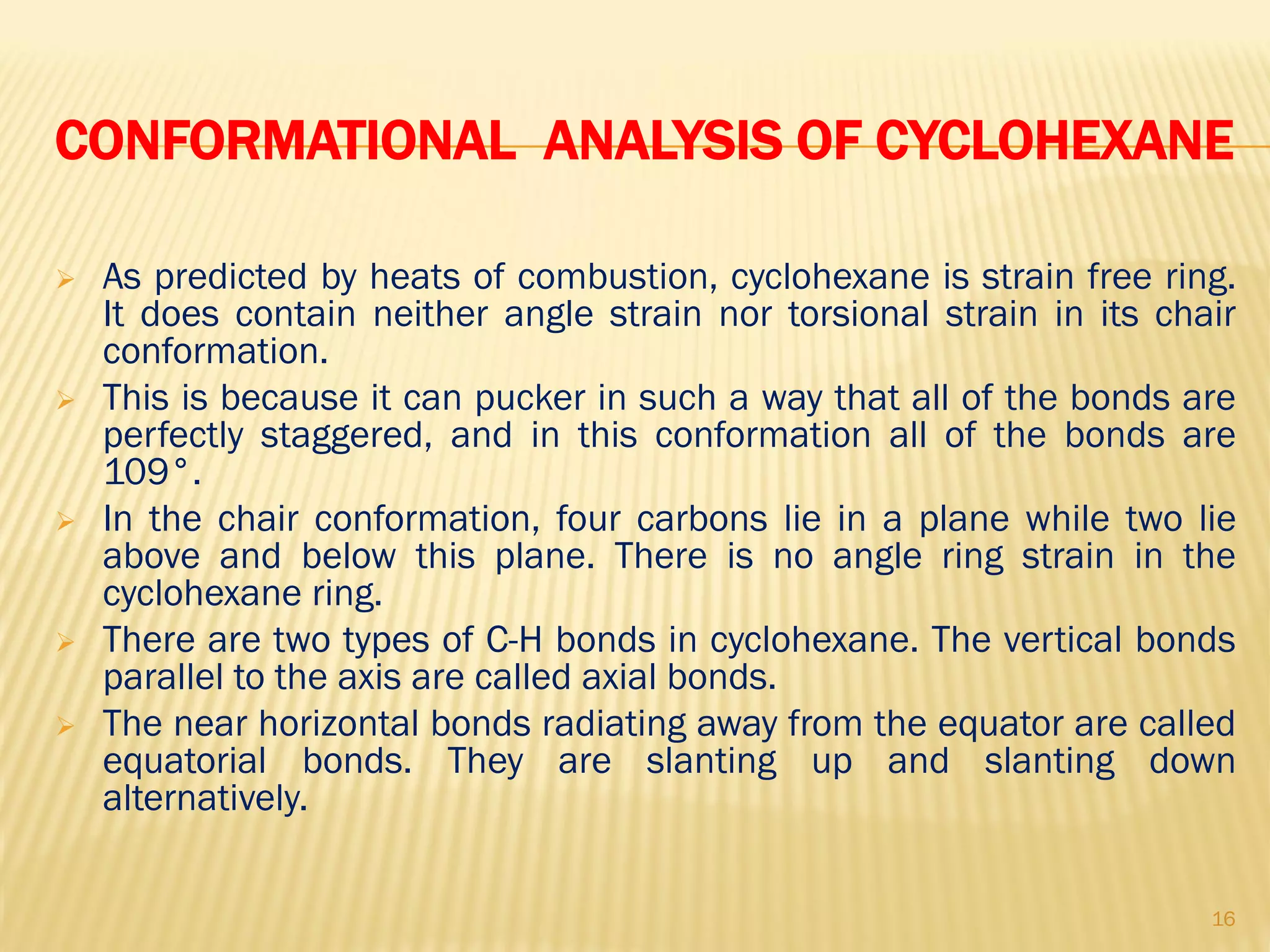
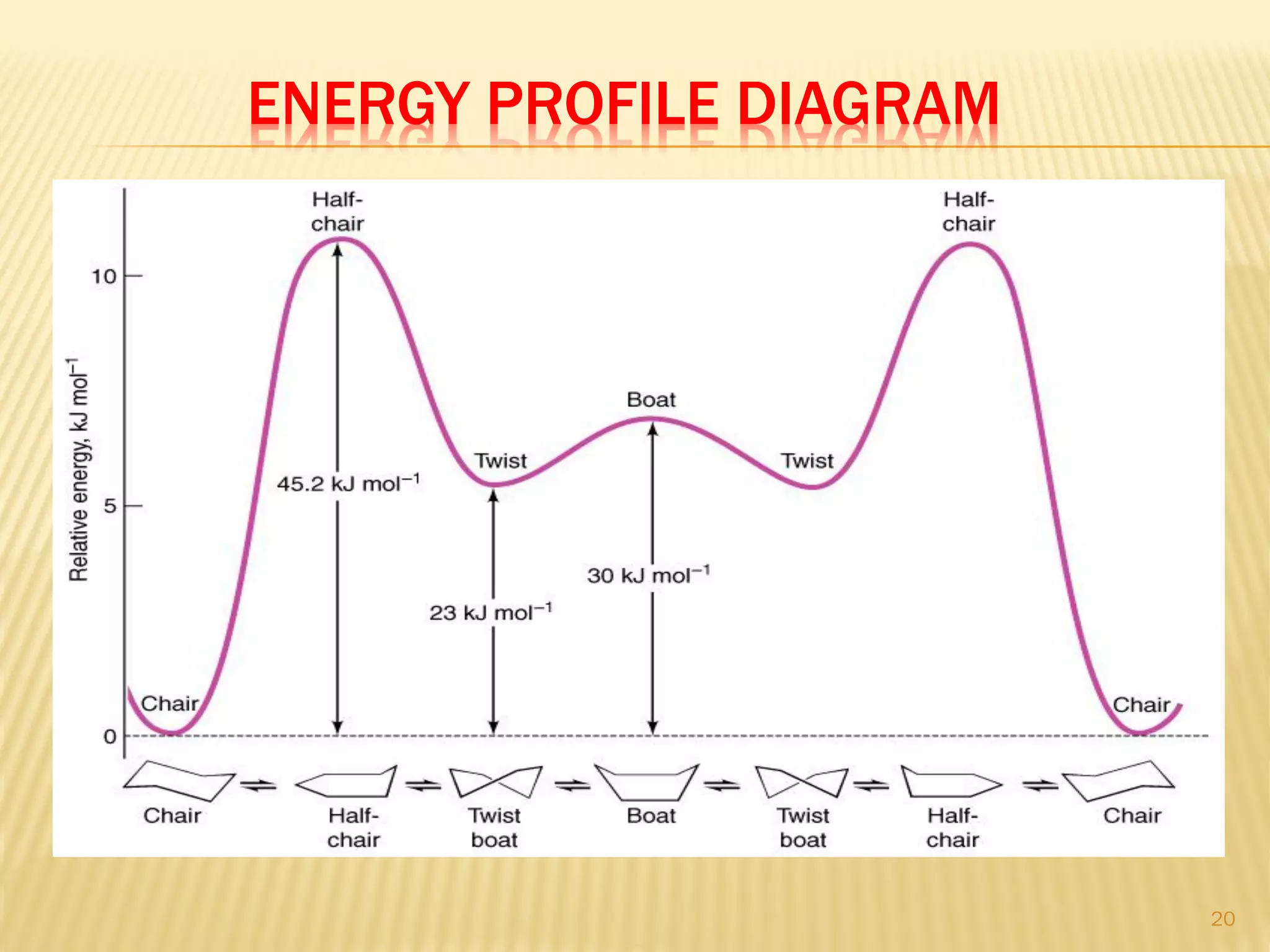
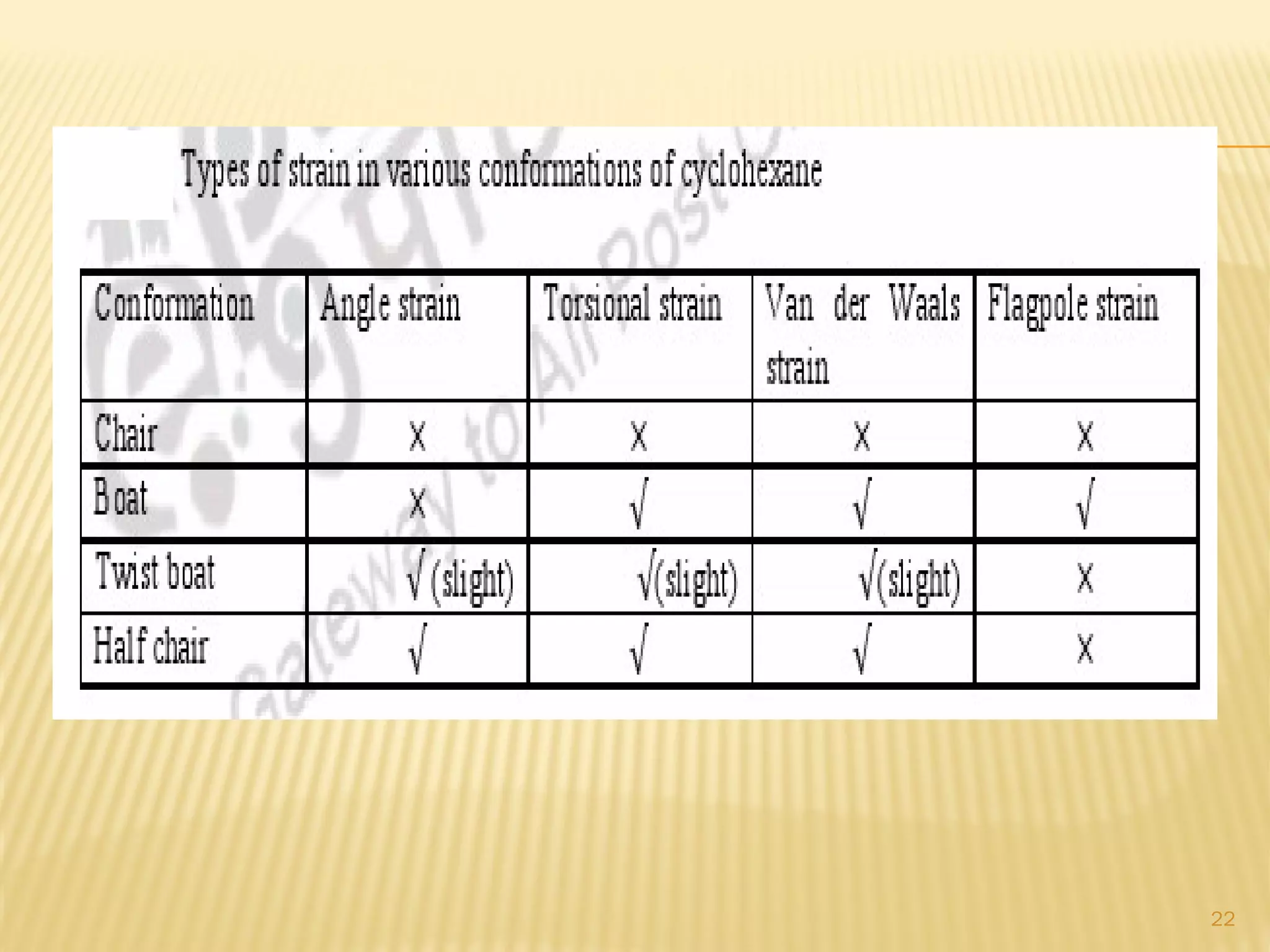
1) Cyclohexane is the most stable cycloalkane due to its chair conformation, which avoids angle and torsional strain through staggered bonding.
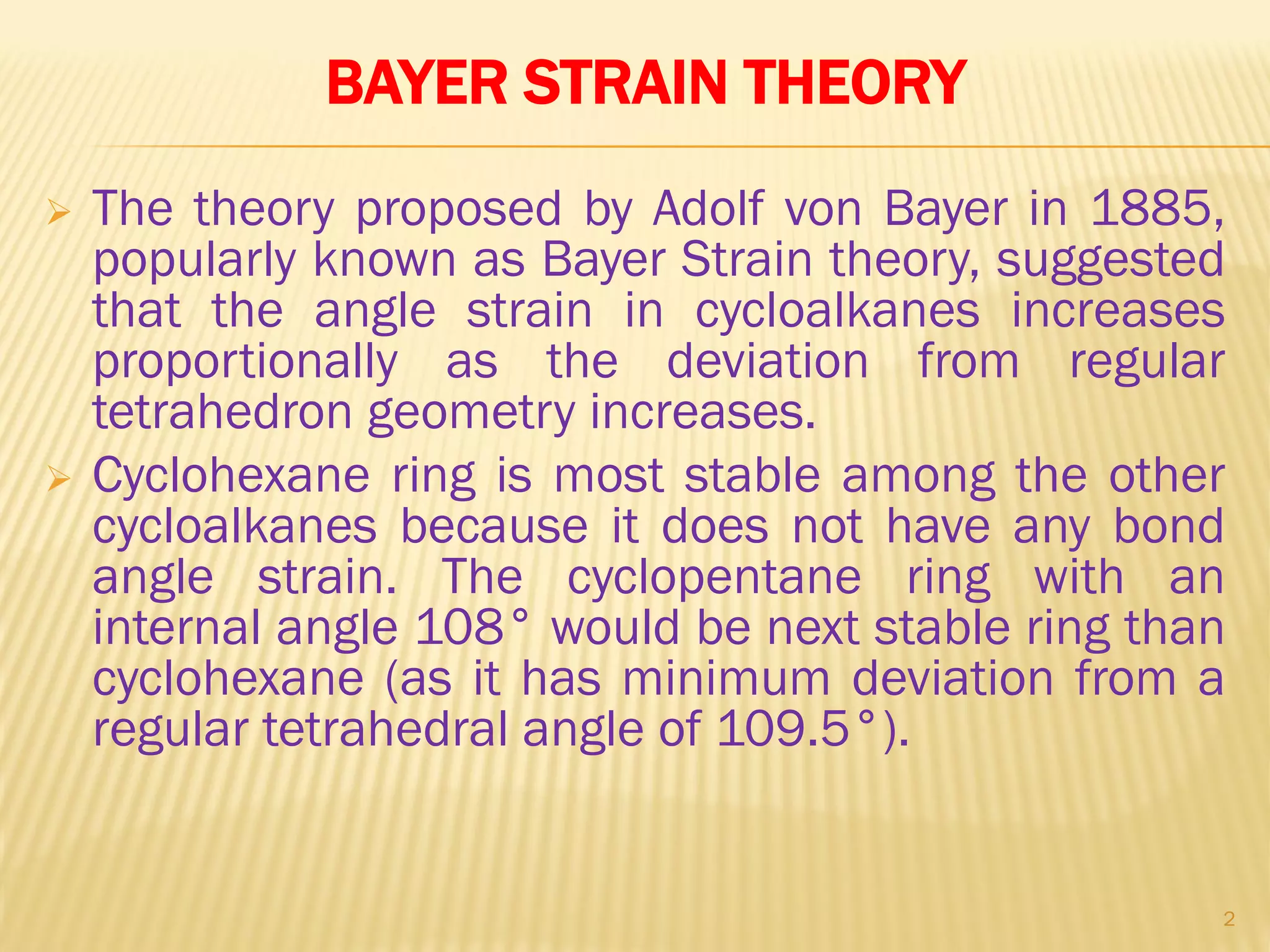
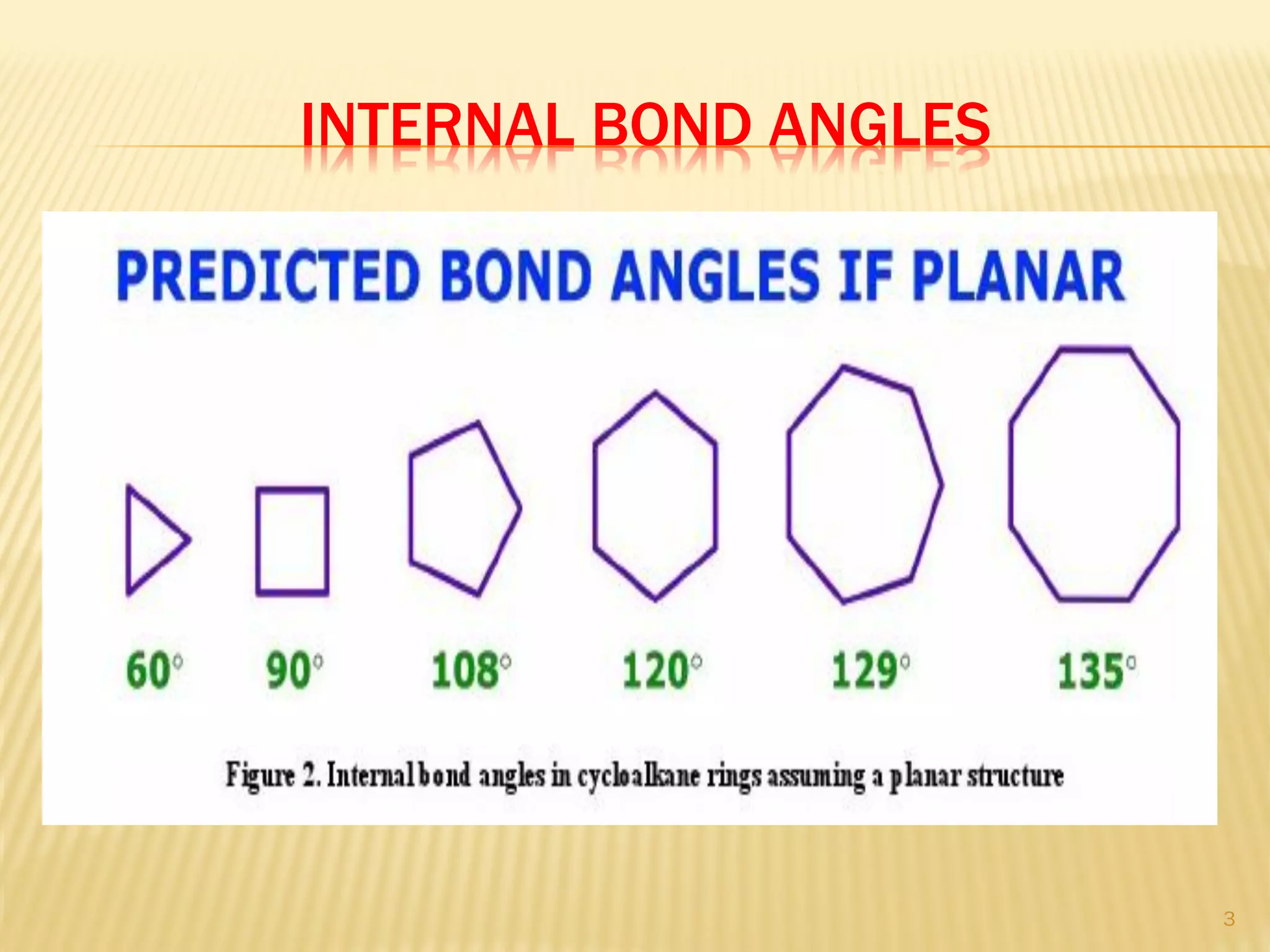
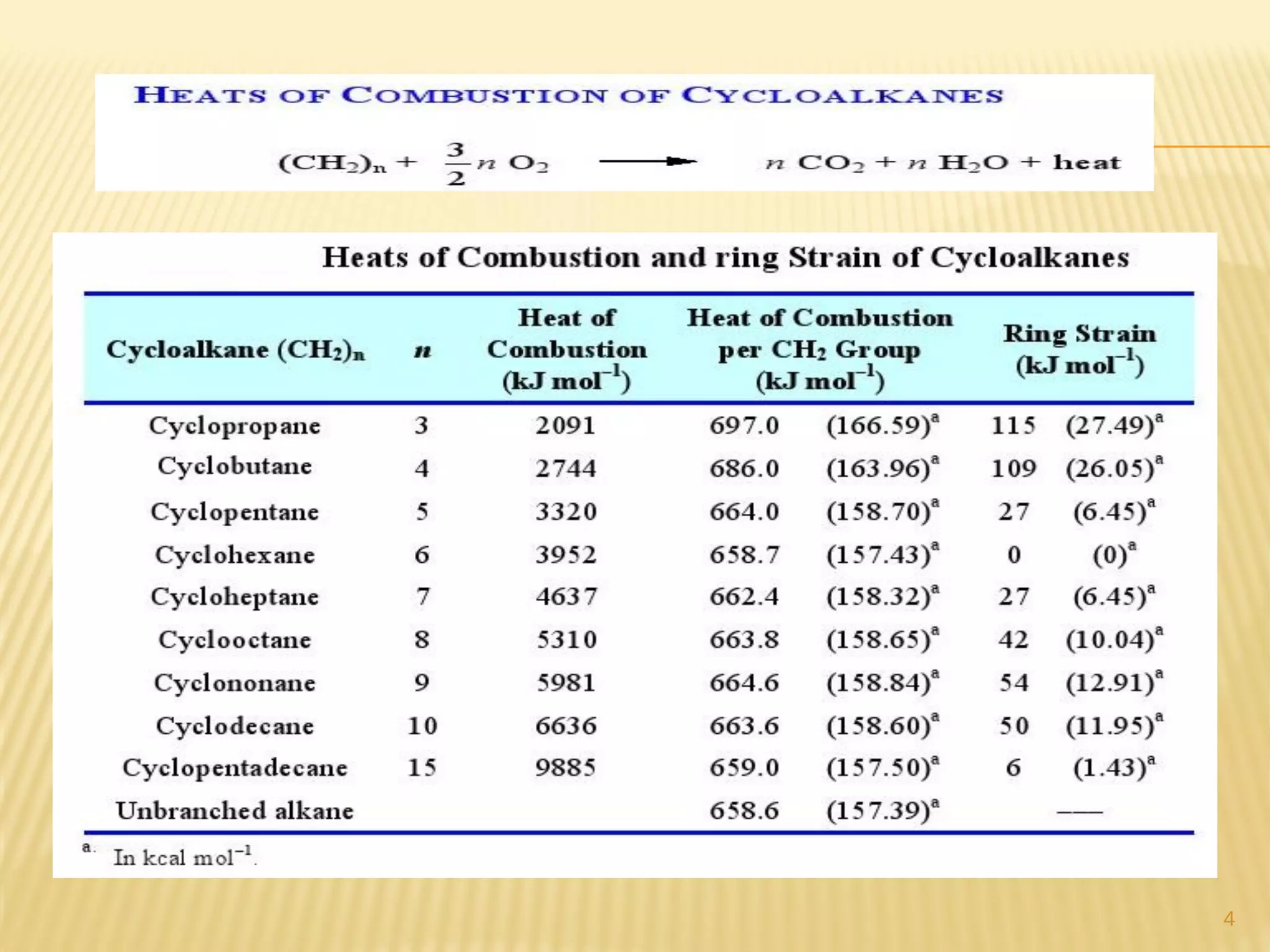
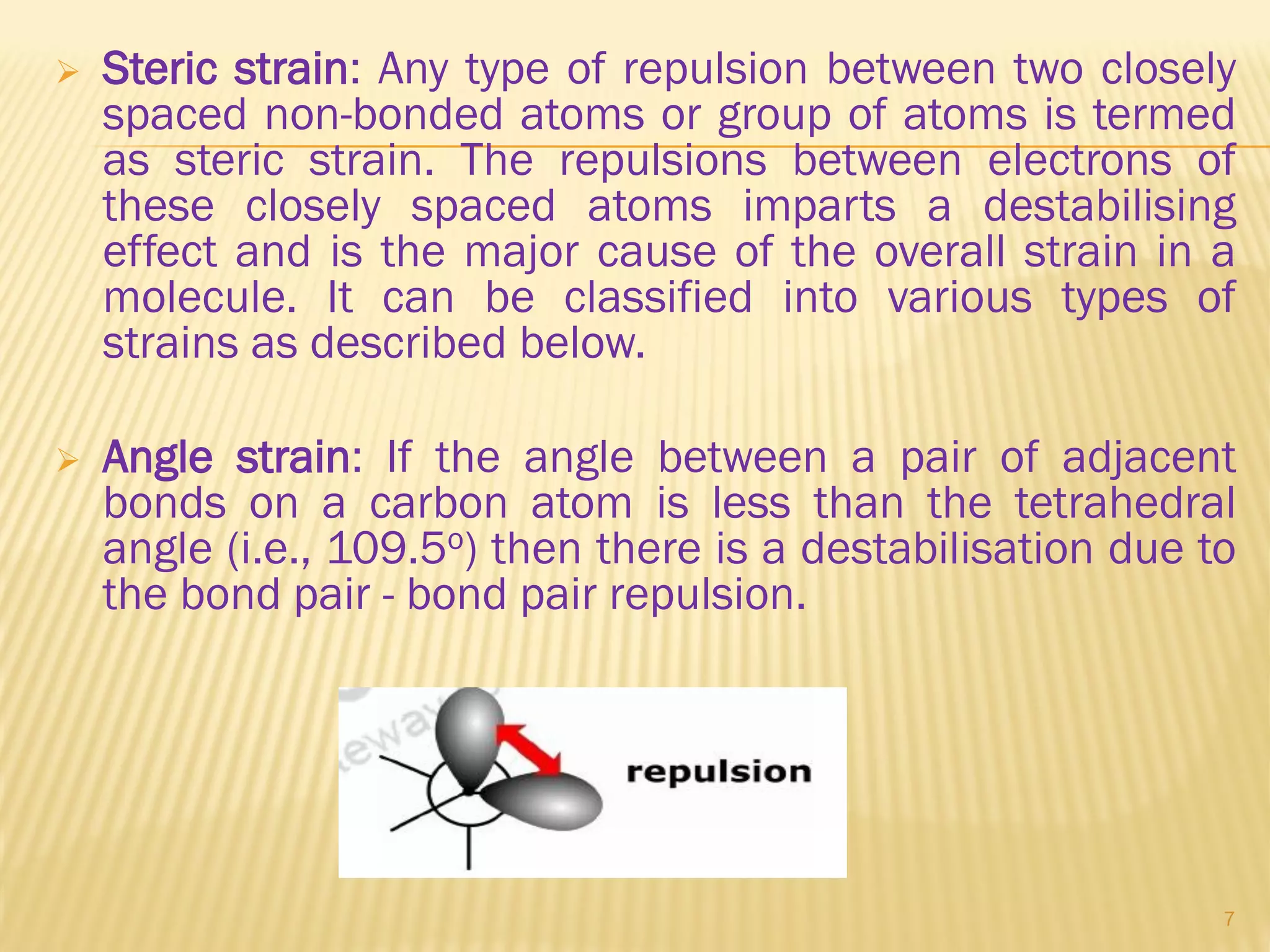
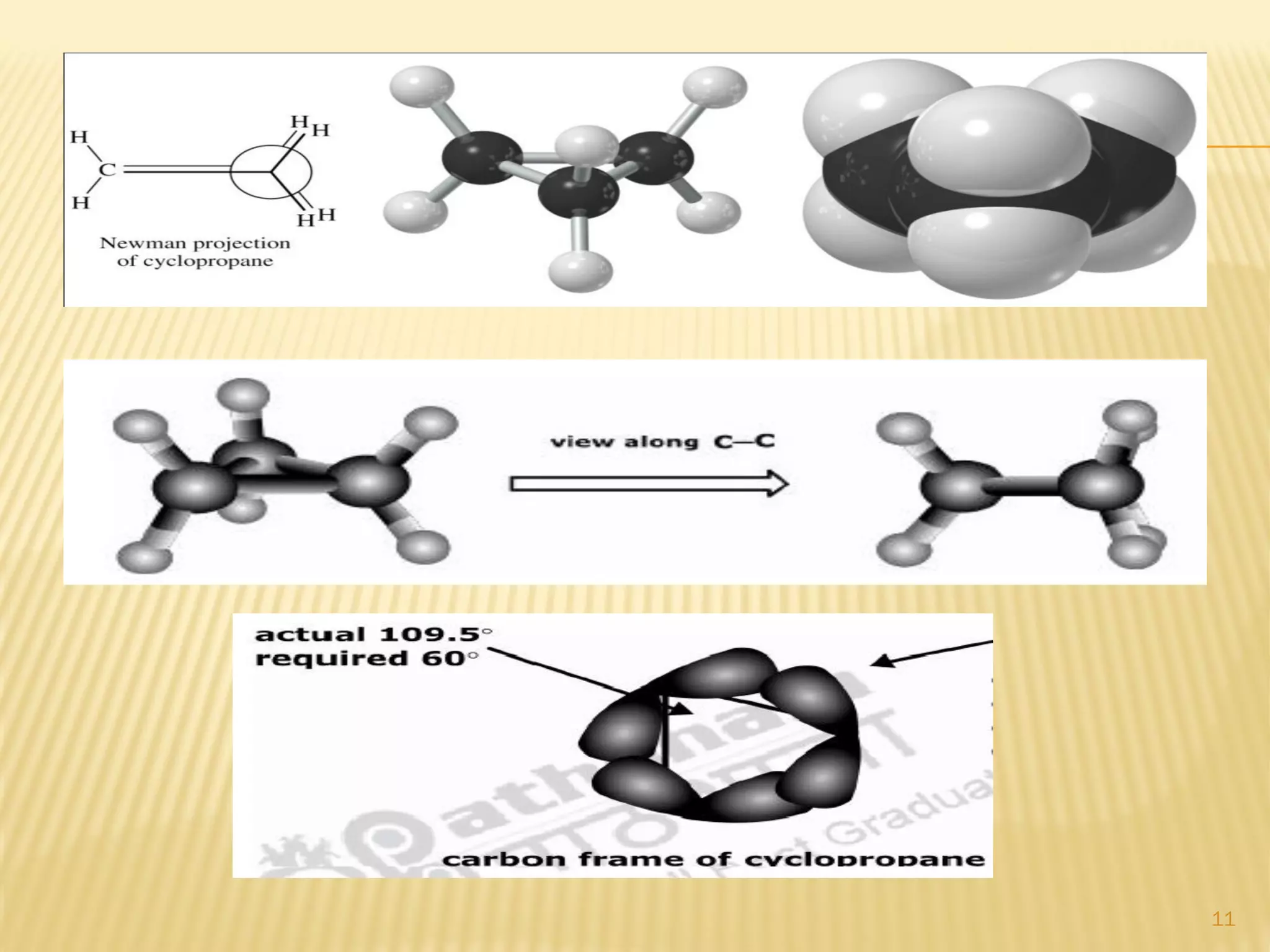
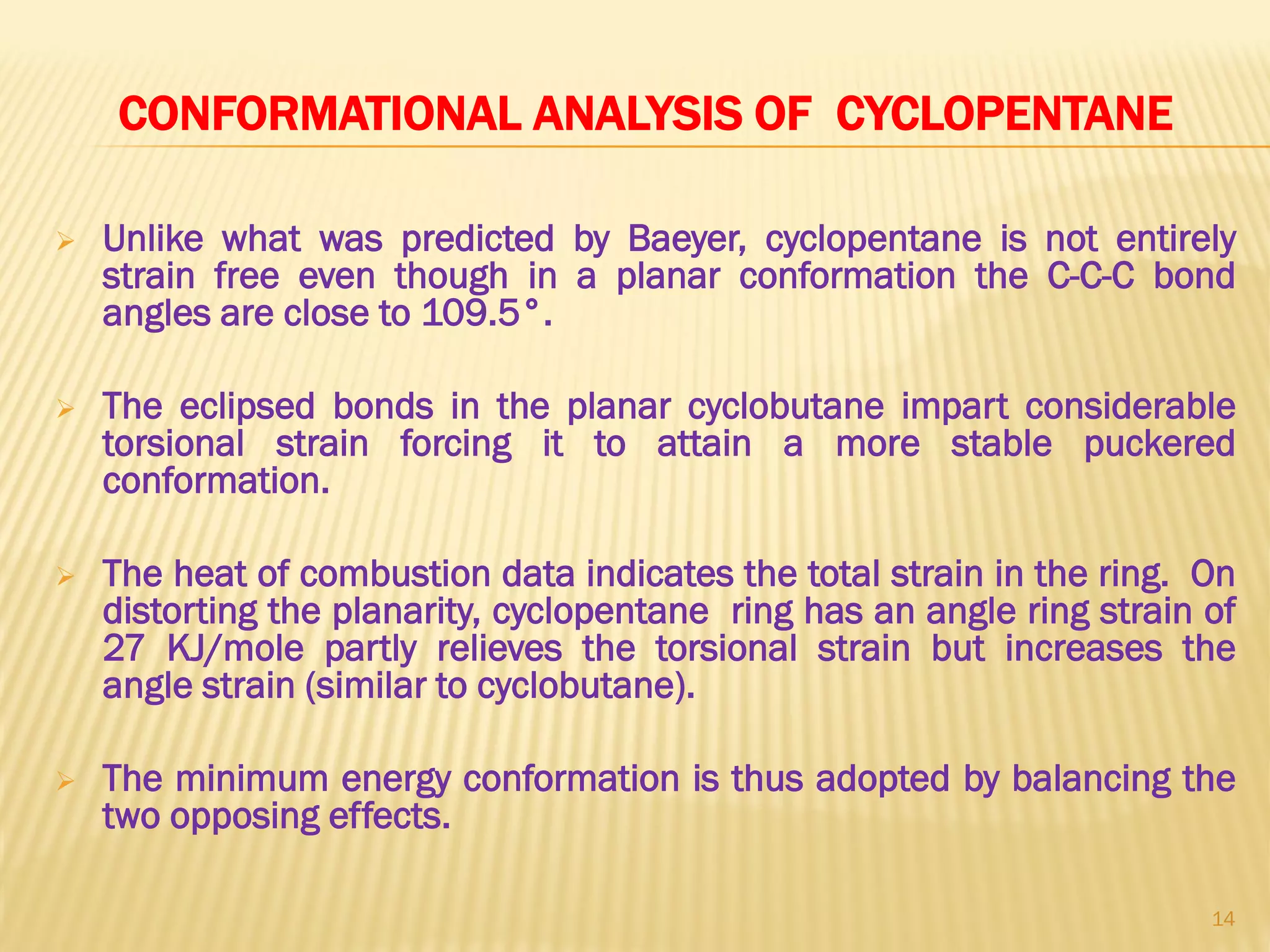
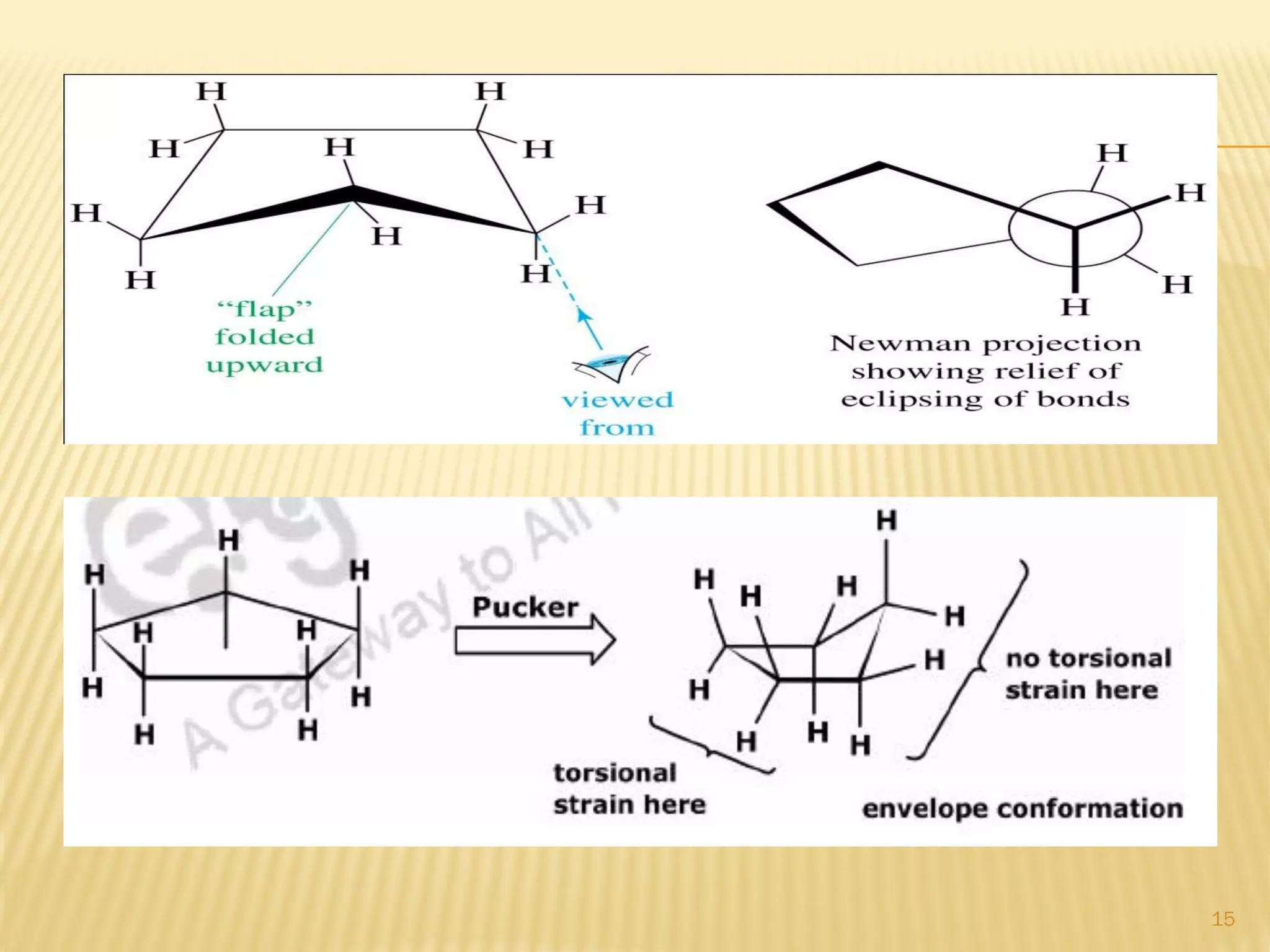
2) Bayer strain theory states that angle strain increases with deviation from the ideal tetrahedral bond angle of 109.5 degrees. Cyclopropane has the most strain due to its 60 degree bond angles.
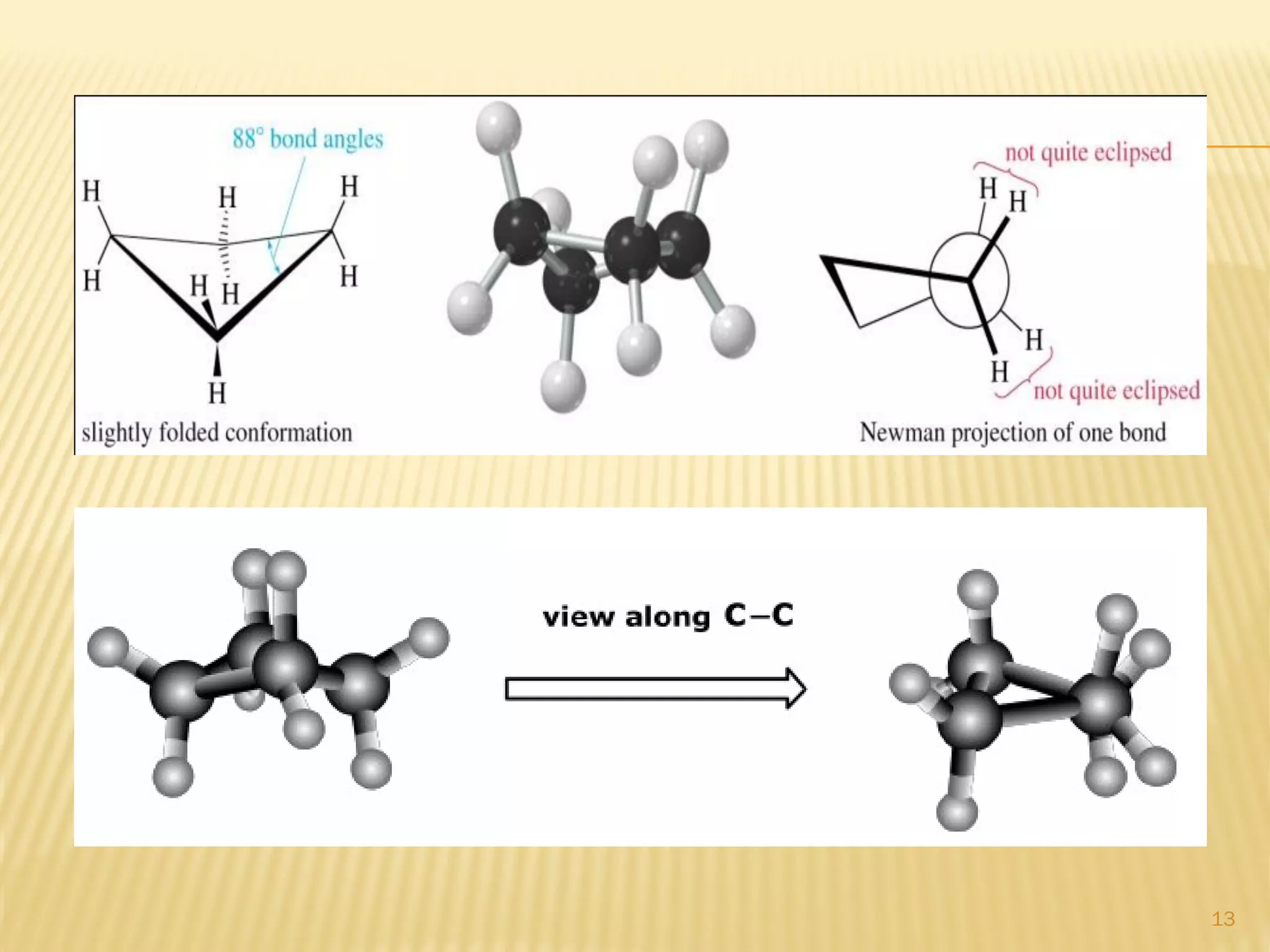
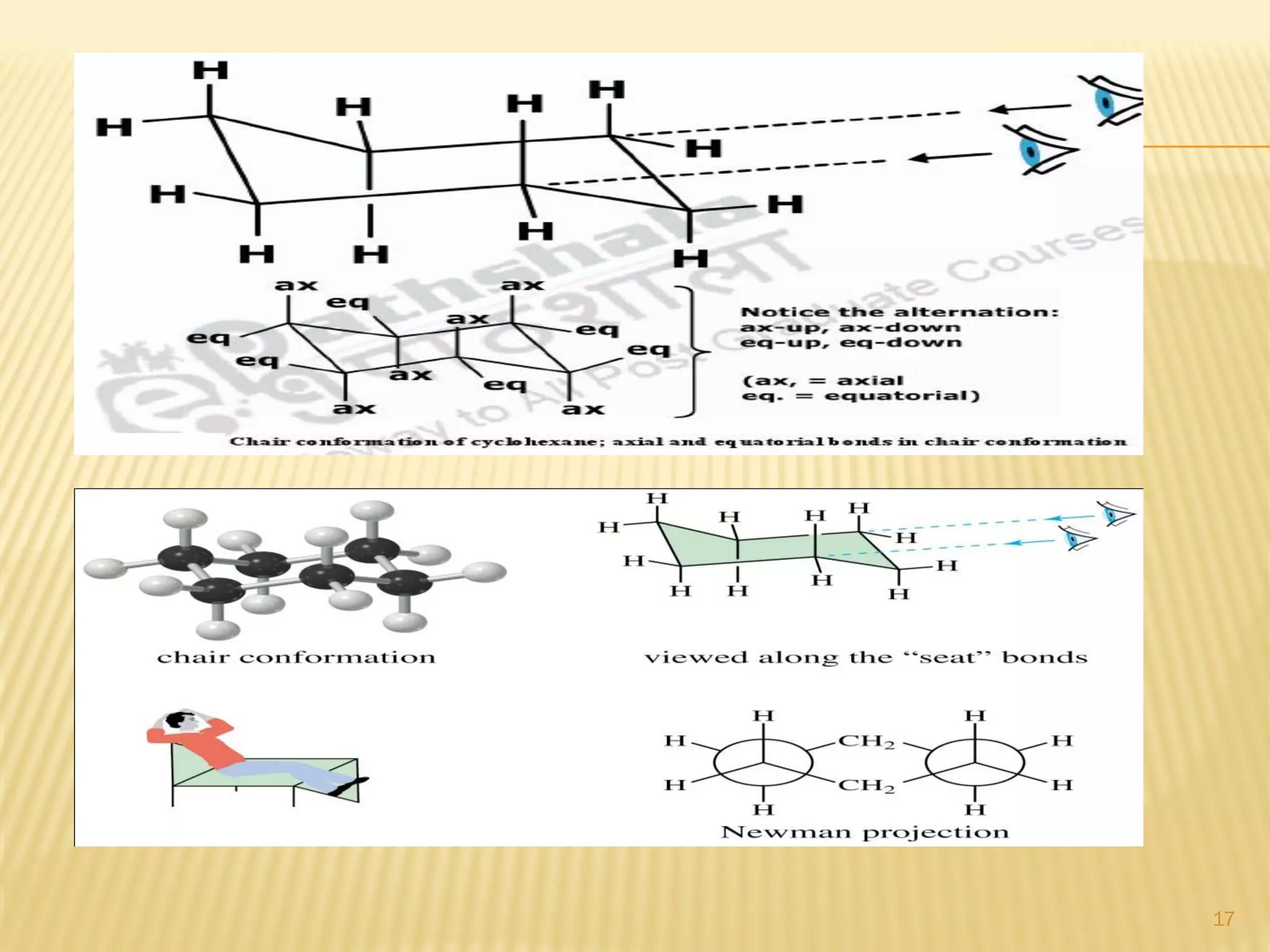
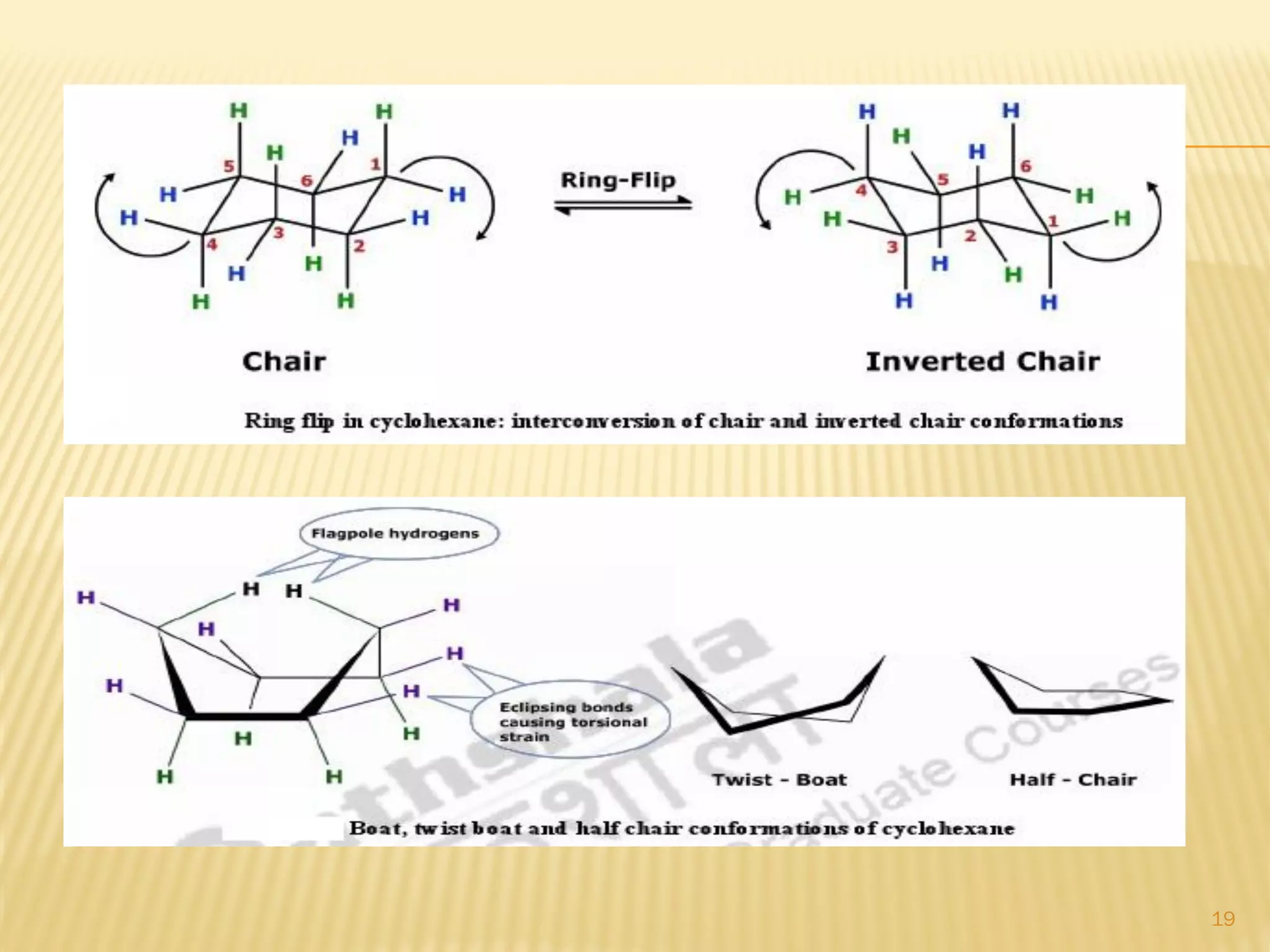
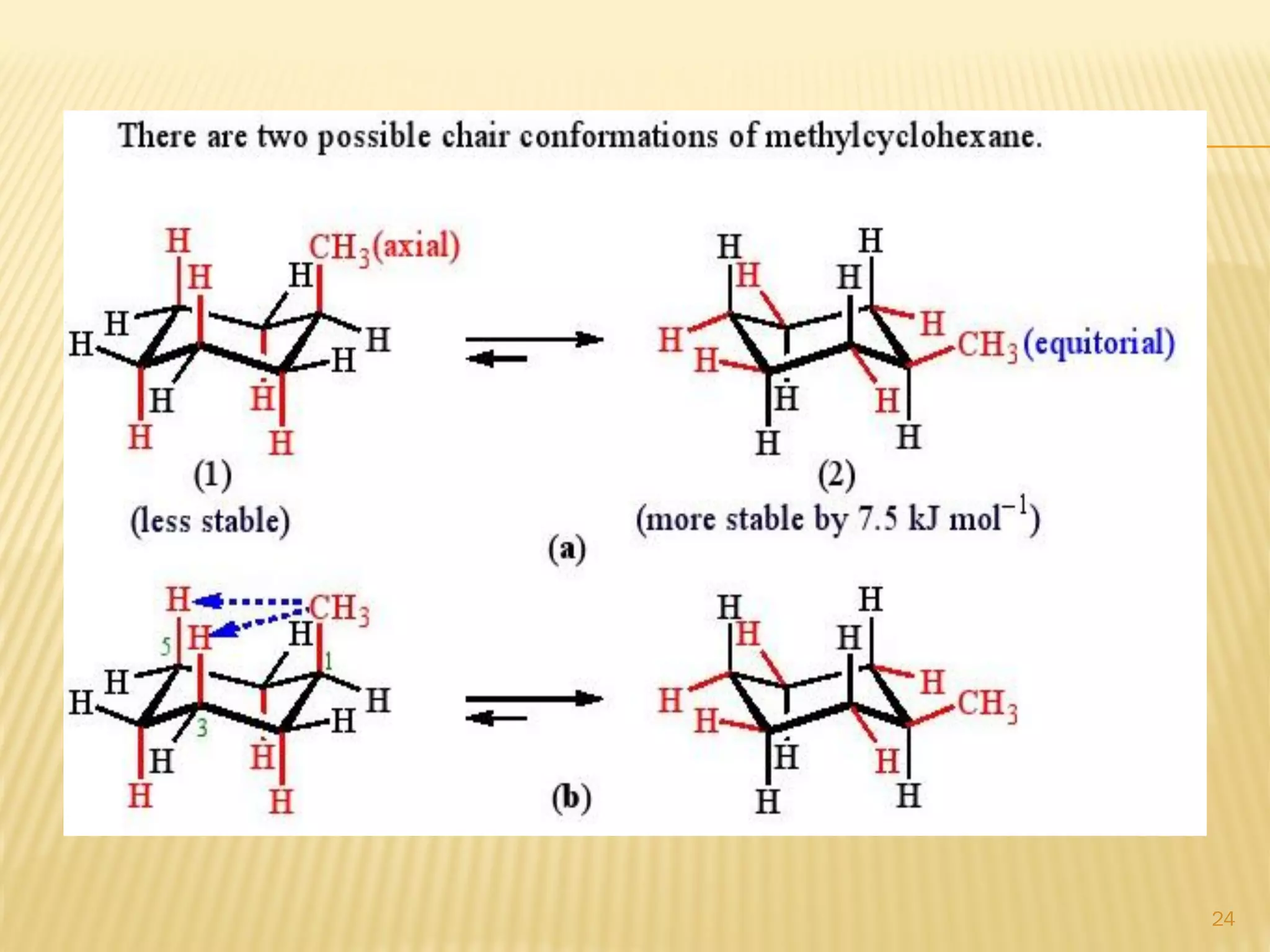
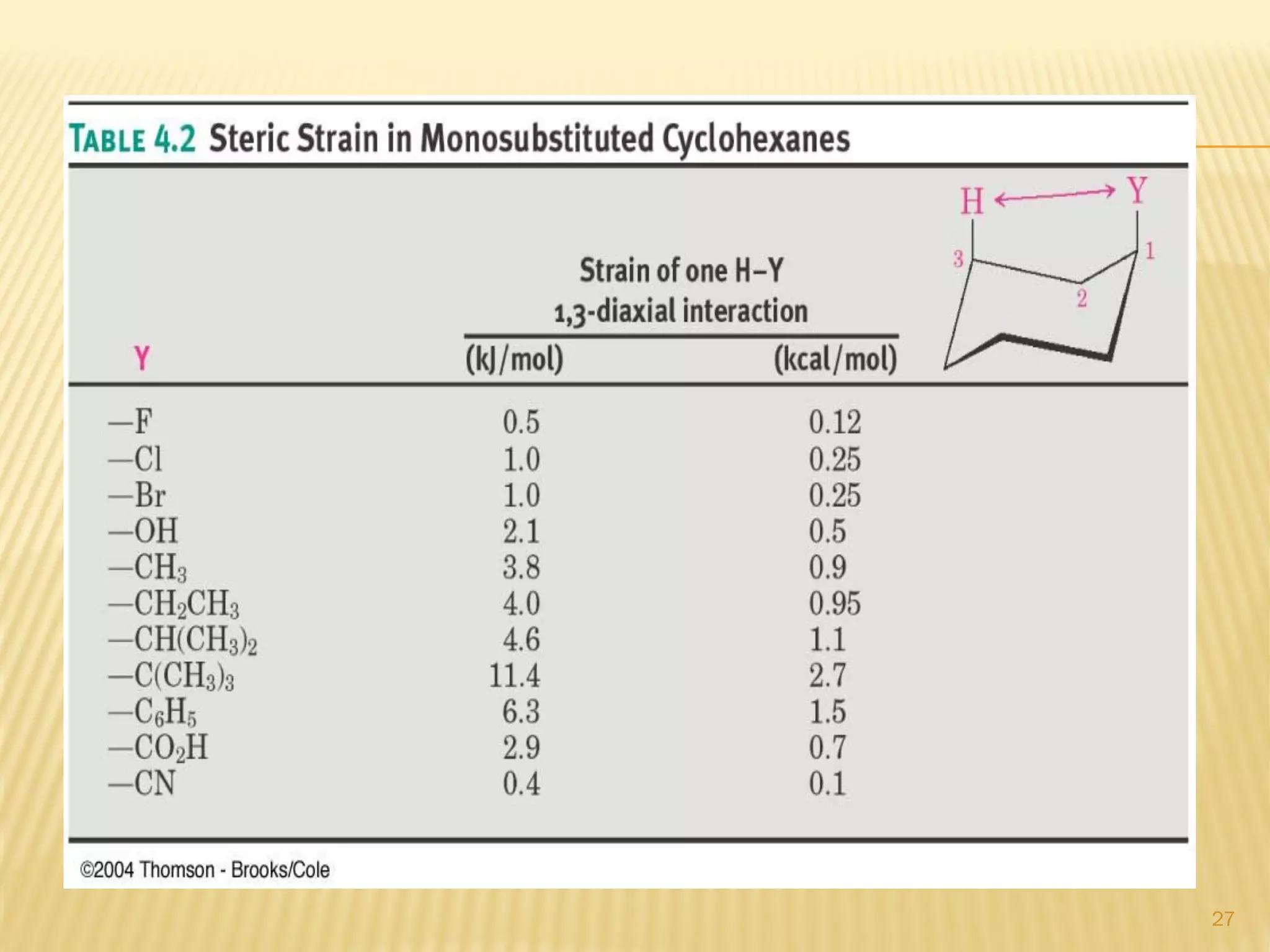
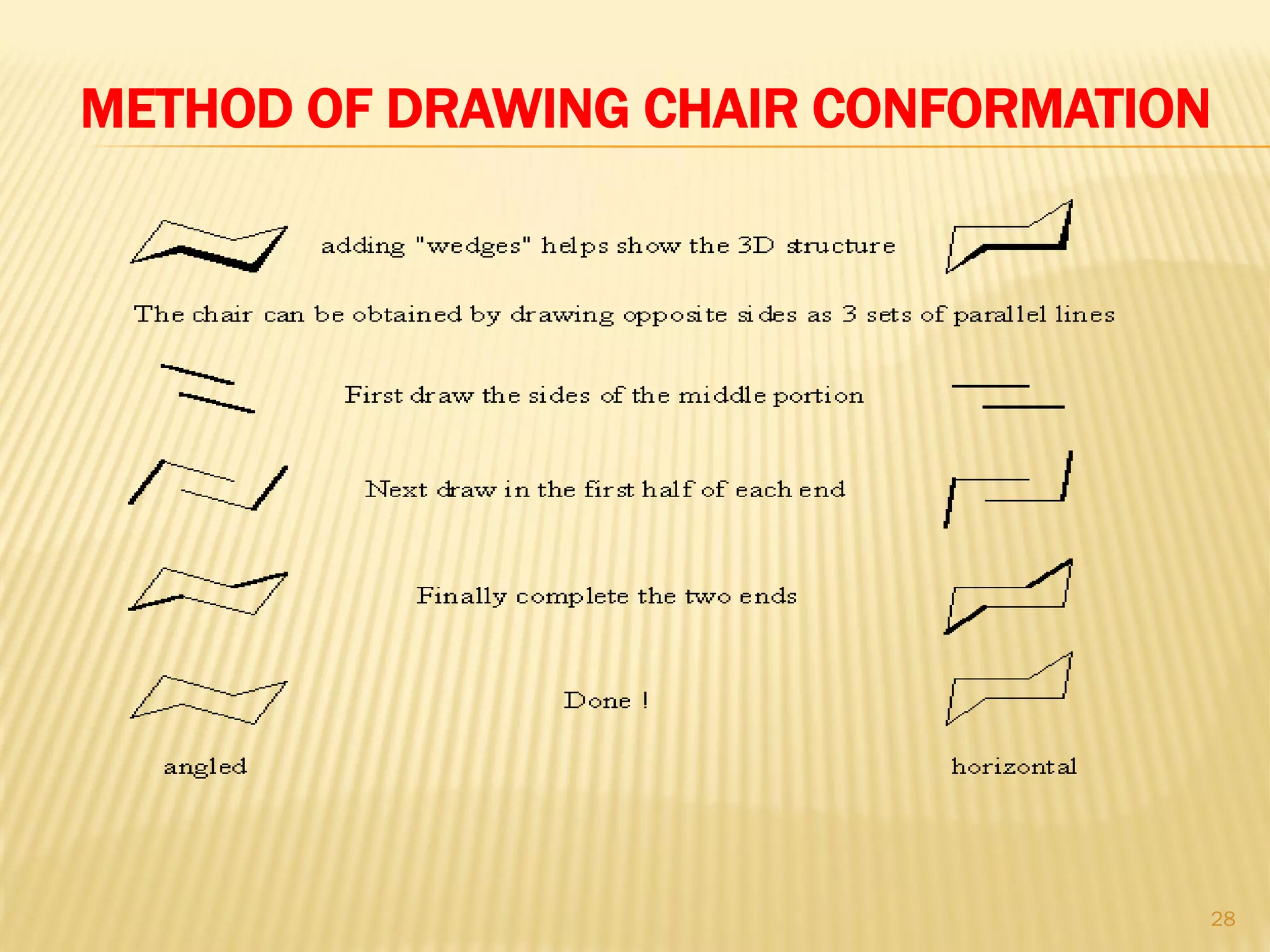
3) In cyclohexane's chair conformation, four carbons lie in a plane while two are above and below, allowing all bonds to be staggered and avoid strain. Monosubstituted cyclohexanes prefer the equatorial position to avoid 1,3-diaxial interactions.