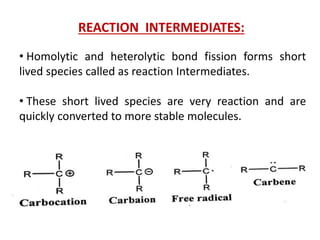
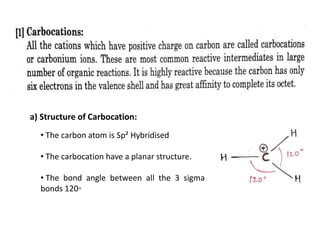
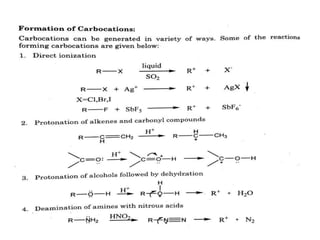
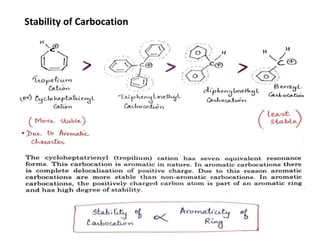
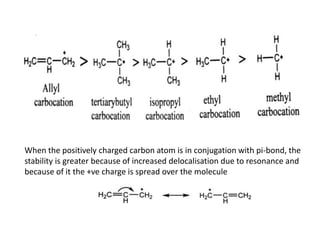
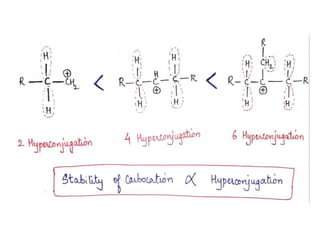
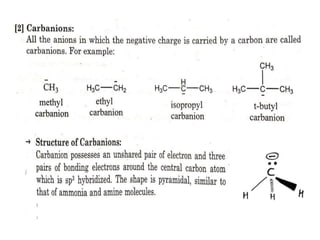
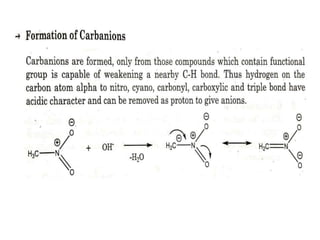
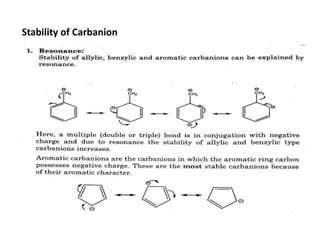
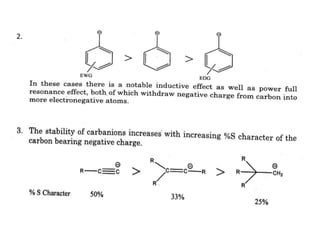
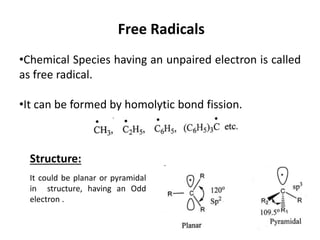
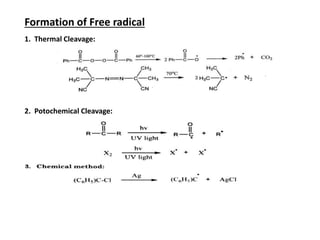
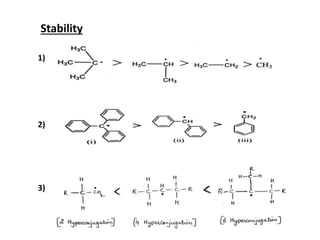
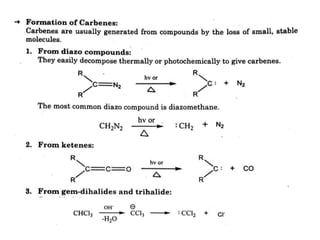
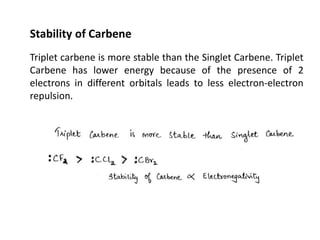
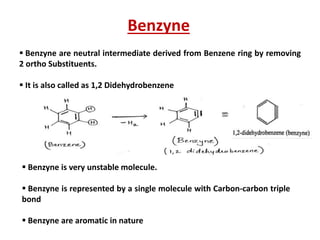
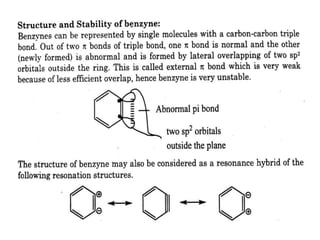
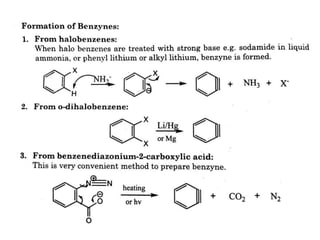
The document discusses various reaction intermediates such as carbocations, carbanions, free radicals, carbenes, and benzyne. It highlights the characteristics, structures, and stability factors of these intermediates, noting that carbocations are planar with a bond angle of 120°, free radicals have unpaired electrons, and triplet carbenes are more stable than singlet carbenes. Additionally, it describes benzyne as an unstable aromatic intermediate derived from benzene with a carbon-carbon triple bond.