Complexometric titration involves titrating a metal ion with a complexing agent like EDTA. Magnesium sulfate can be estimated by direct titration with EDTA in the presence of ammonia-ammonium chloride buffer using an indicator. The magnesium ions form a complex with EDTA until the equivalence point is reached, indicated by a color change of the indicator. This direct titration method provides an accurate determination of the magnesium content in magnesium sulfate.


![• Complexometry : is the type of volumetric
analysis involving the formation of complexes
which are slightly ionized in solution, like weak
electrolyte and sparingly soluble salt.
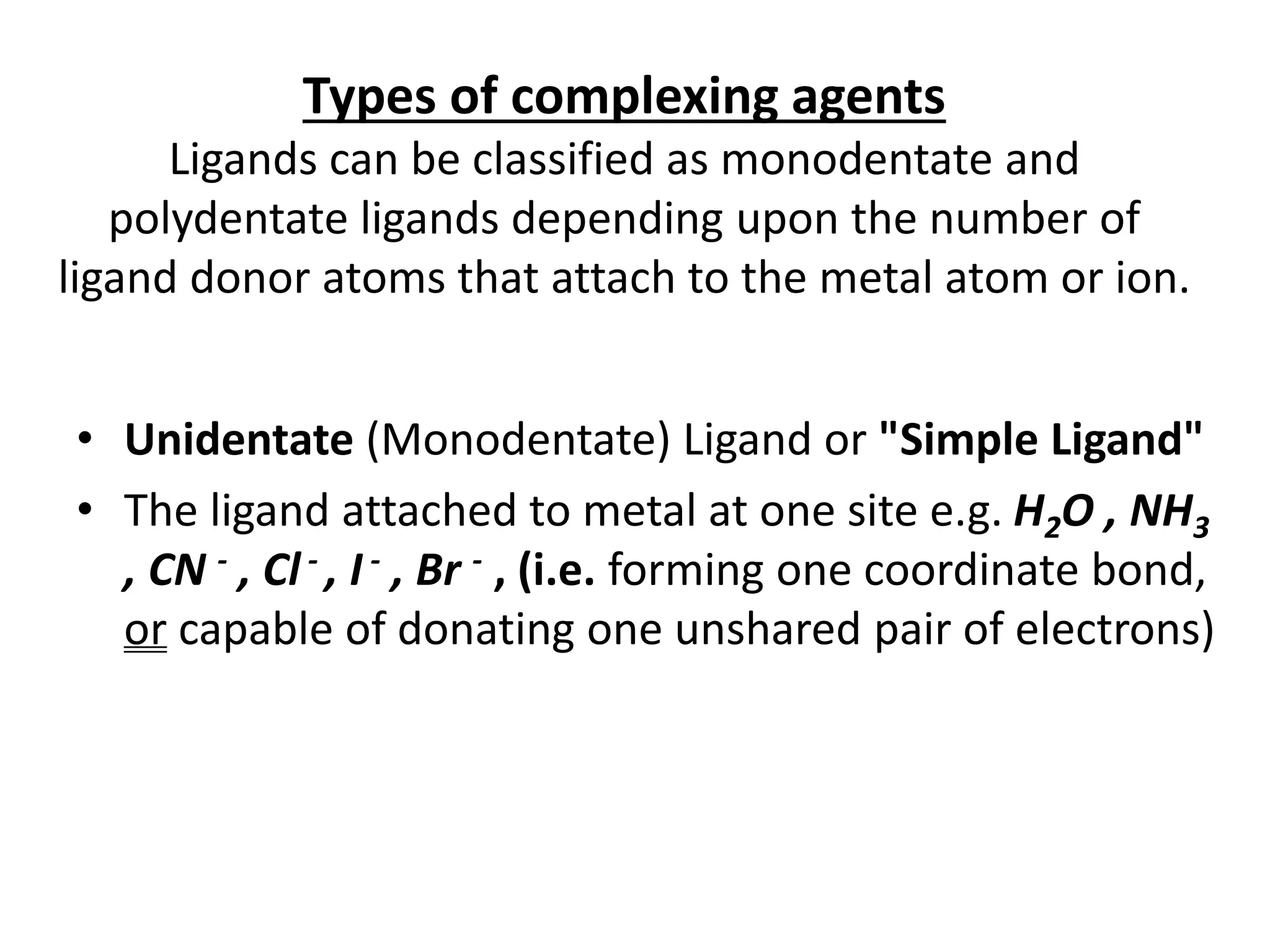
• Complex is formed by the reaction of metal
ion (Mn+) with either an anion e.g. [Ag(CN)2]-
or neutral molecule e.g. [Ag(NH3)2]+
• The metal ion is known as Central metal
atom.
• The anion or neutral molecule is known as
Ligand (L)](https://image.slidesharecdn.com/complexometrictitration-181006073935-240223100721-baea88c9/75/complexometric-titration-for-b-pharm-students-5-2048.jpg)
![• M+ + L ML
• Ag+ + 2 CN- [Ag(CN)2]-
• Cu2+ + 4 CN- [Cu(CN)4]2-
• Ag+ + 2 NH3 [Ag(NH3)2]+
• Central metal atom = acts as Lewis acid
(electron acceptor)
• Ligand = acts as Lewis base (electron donor)
• Coordinate bond (dative) = The bond formed
between central metal atom (ion) (acceptor)
and the Ligand (donor)](https://image.slidesharecdn.com/complexometrictitration-181006073935-240223100721-baea88c9/75/complexometric-titration-for-b-pharm-students-6-2048.jpg)

![Coordination number = The no. of coordinate
bonds formed to a metal ion by its ligands.
• The charge of a complex is the algebraic sum
of the charges of the central ion and ligand ..
e.g.
• [Ag(CN)2] - Ag+ + 2 CN -
• 1 (+ve) + 2 (-ve) = 1 (-ve)
• e.g. [Fe(CN)6]3- Fe3+ + 6 CN -
3 (+ve) + 6 (-ve) = 3 (-ve)
• The higher the valence of metal ion the more
stable the complex e.g.Ferricyanide is more
stable than Ferrocyanide](https://image.slidesharecdn.com/complexometrictitration-181006073935-240223100721-baea88c9/75/complexometric-titration-for-b-pharm-students-8-2048.jpg)



![Complexing agents are different
types
1.Neutral molecules: neutral molecules having lone pair of
electron act as complexing agent
Example: ammonia forms cuprammonium complex with
copper [Cu(NH3)4]2+
2. Group whose proton can be easily replaced such as –
COOH, phenolic and enolic OH.
3.Sequestring agents having group like COOH, NH2 and OH.
Example of complexing agent: EDTA, Dimethylglyoxime,
salicylaldoxime](https://image.slidesharecdn.com/complexometrictitration-181006073935-240223100721-baea88c9/75/complexometric-titration-for-b-pharm-students-13-2048.jpg)












![• Demasking agent : is the process in which masking substance
reveres back to its ability to take part in the reaction.
- are reagents which regain the ability of masked ion to enter
the reaction with ind. and EDTA.
• Example:
The masking by CN– can be removed by:
- mixture of formaldehyde – acetic acid
- on addition of demasking agent to [Zn(CN)4]2- , Zn is liberated
and titrated.
• [Zn(CN)4]2- + 4 HCHO + 4 CH3COOH
(less stable) CN
• Zn2+ + 4 CH2 + 4 CH3COO-
• OH
Cyanohydrin
(more stable)](https://image.slidesharecdn.com/complexometrictitration-181006073935-240223100721-baea88c9/75/complexometric-titration-for-b-pharm-students-26-2048.jpg)




