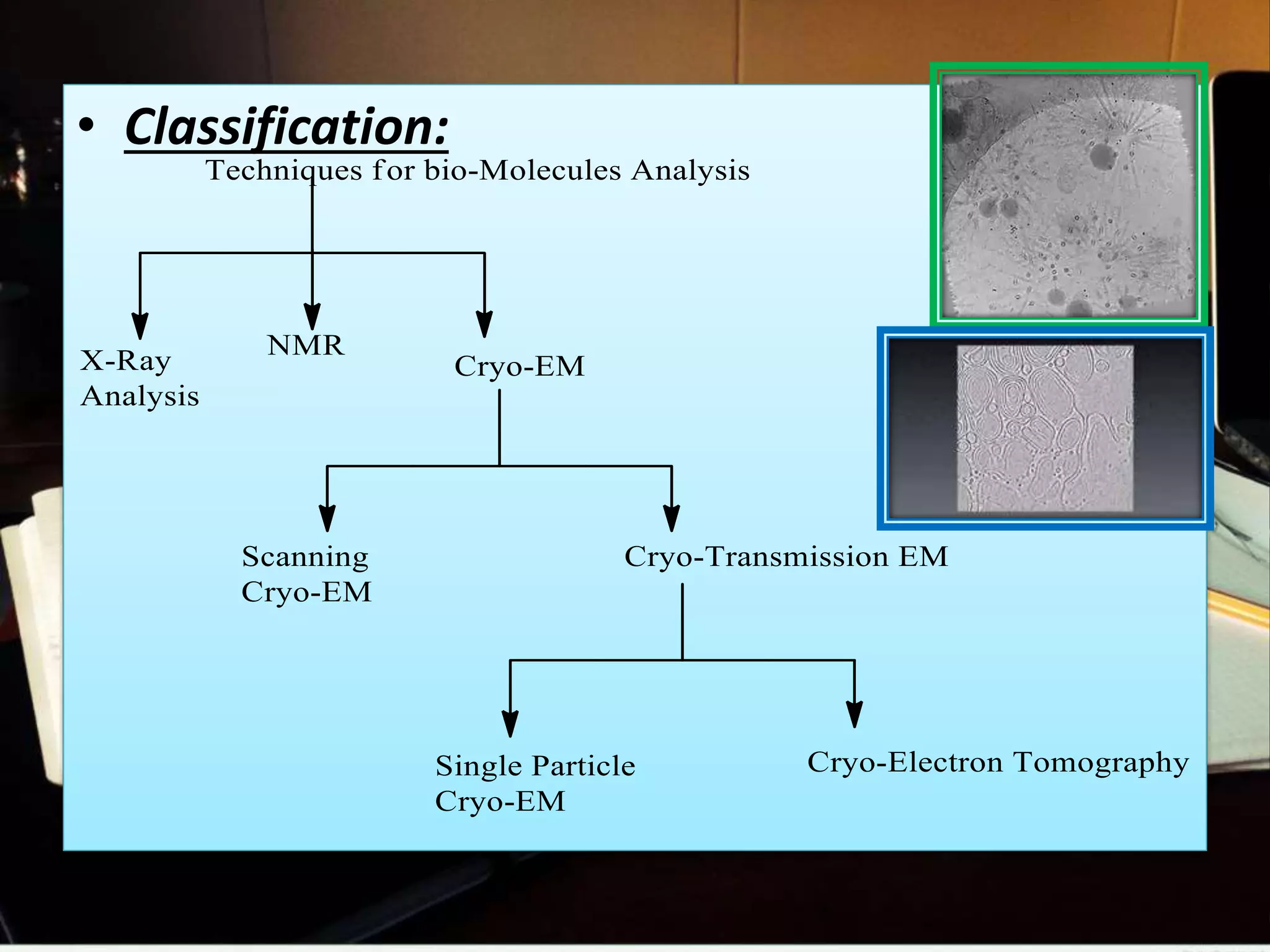
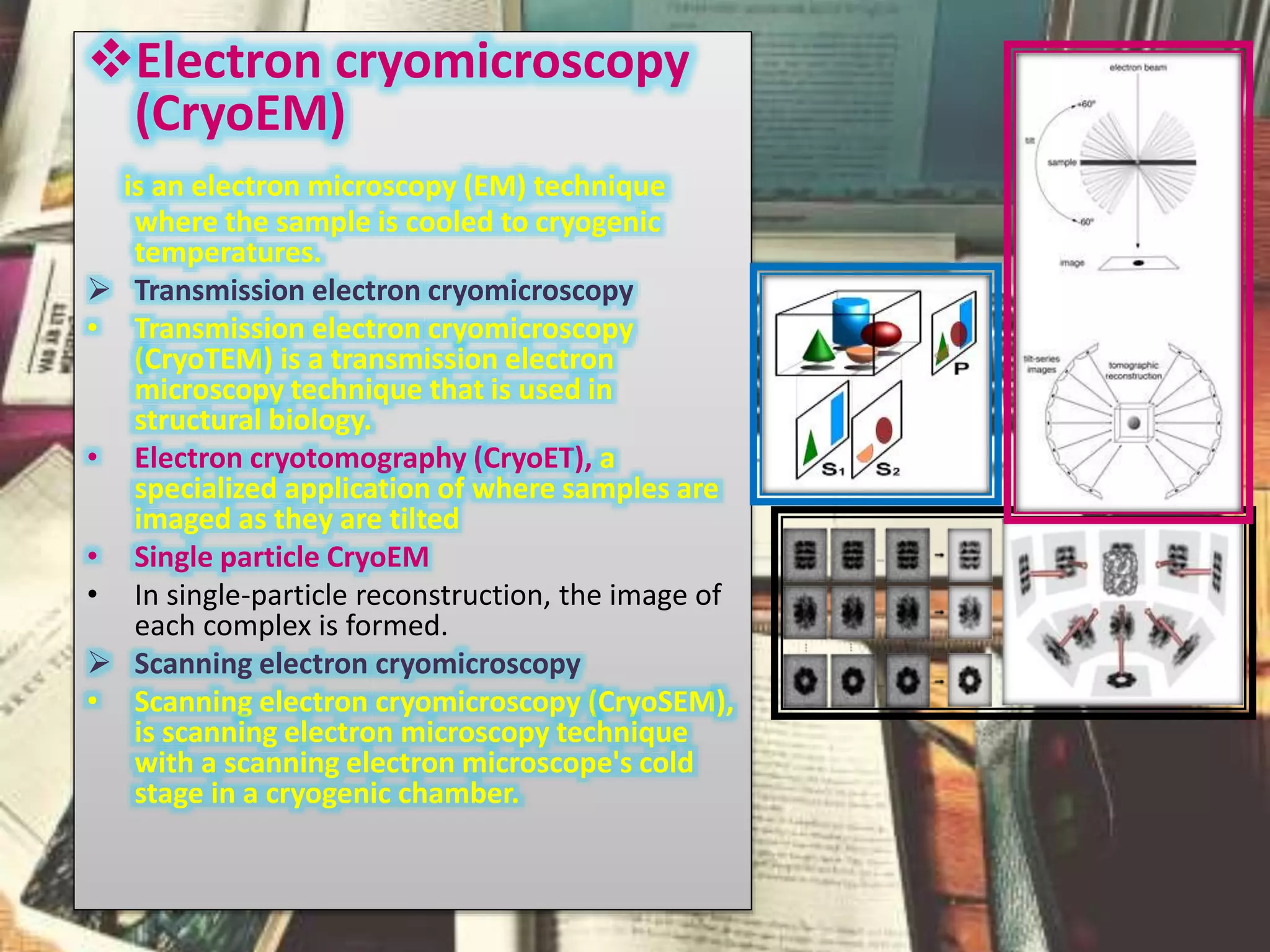
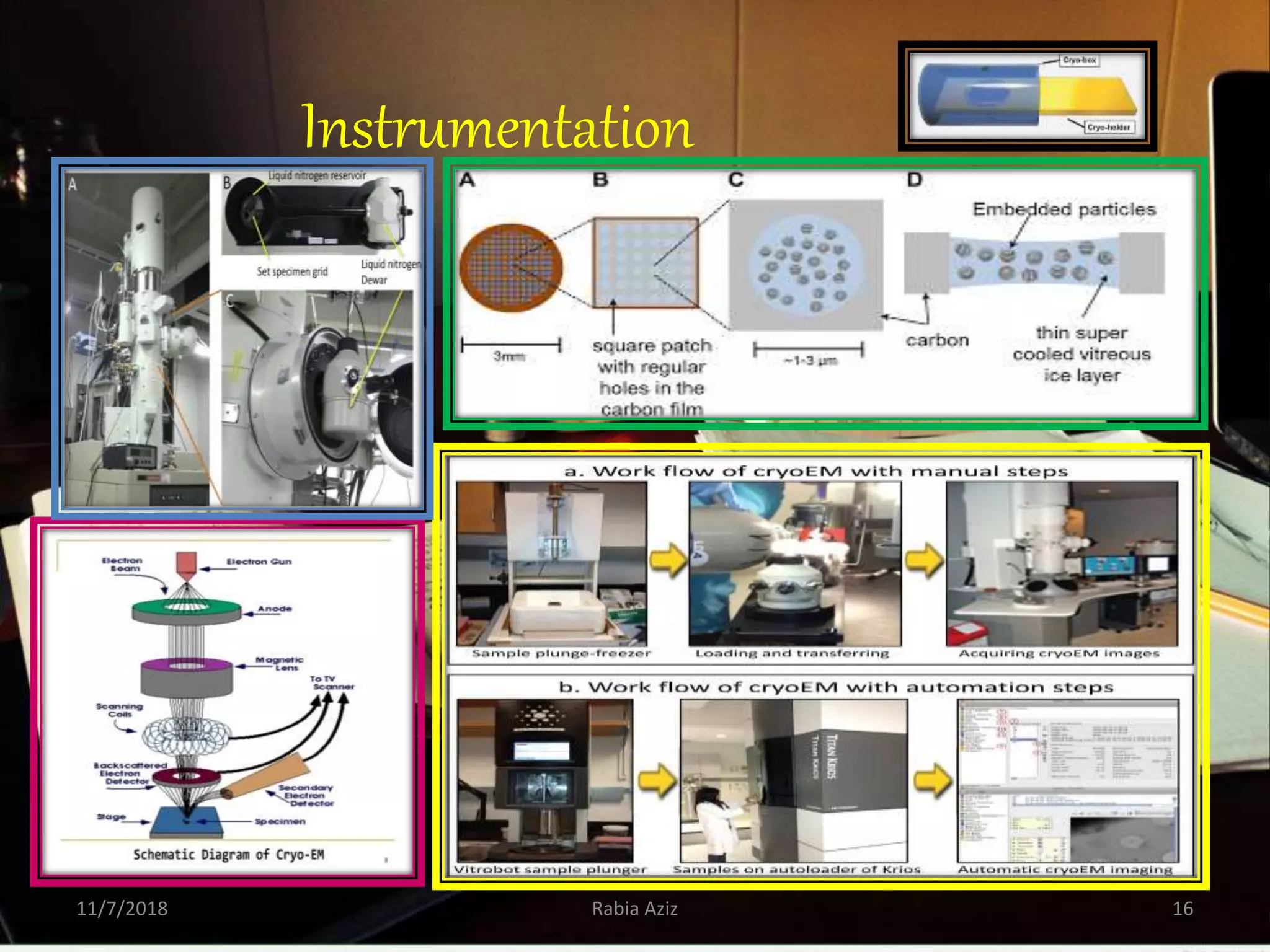
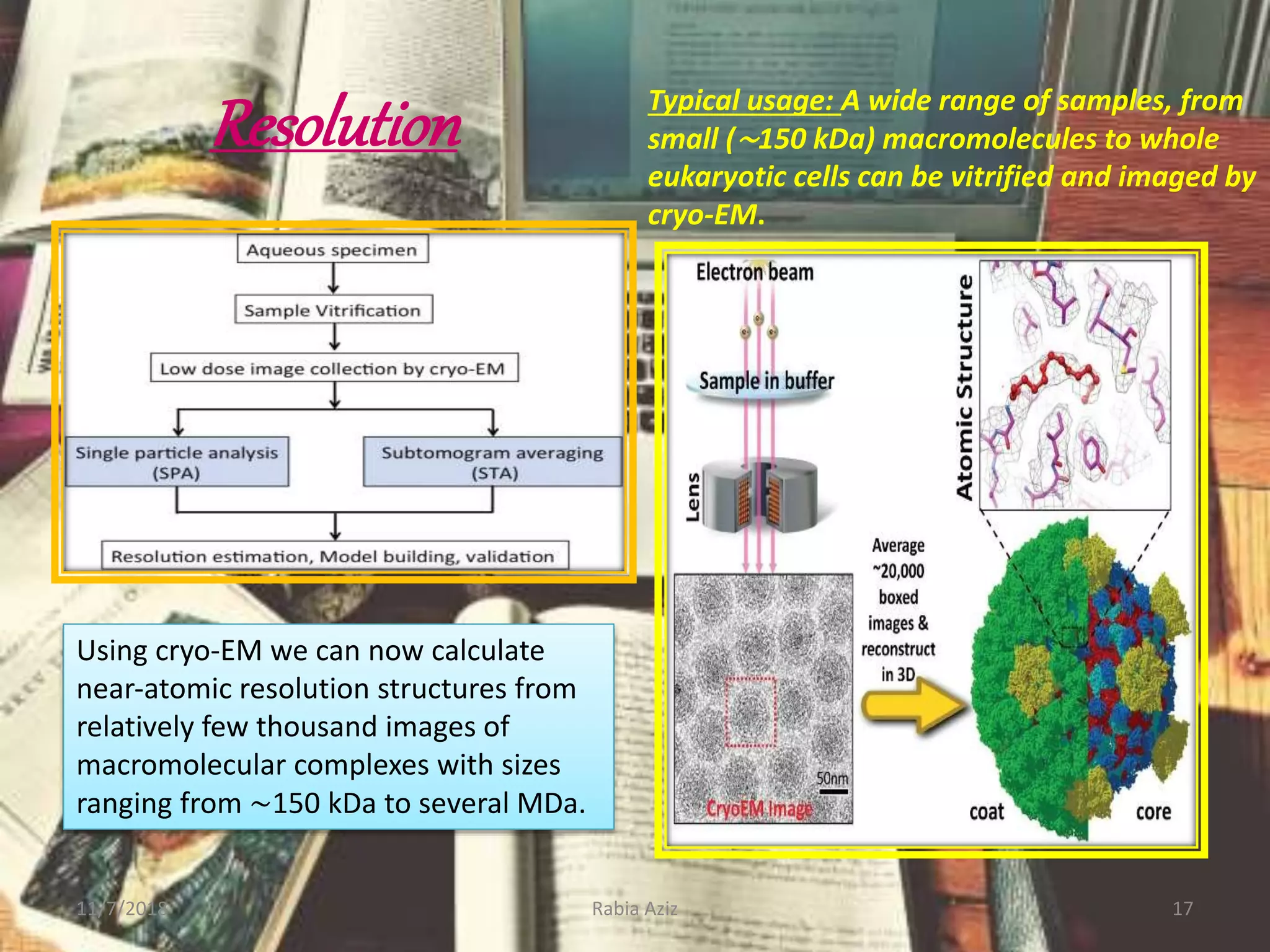
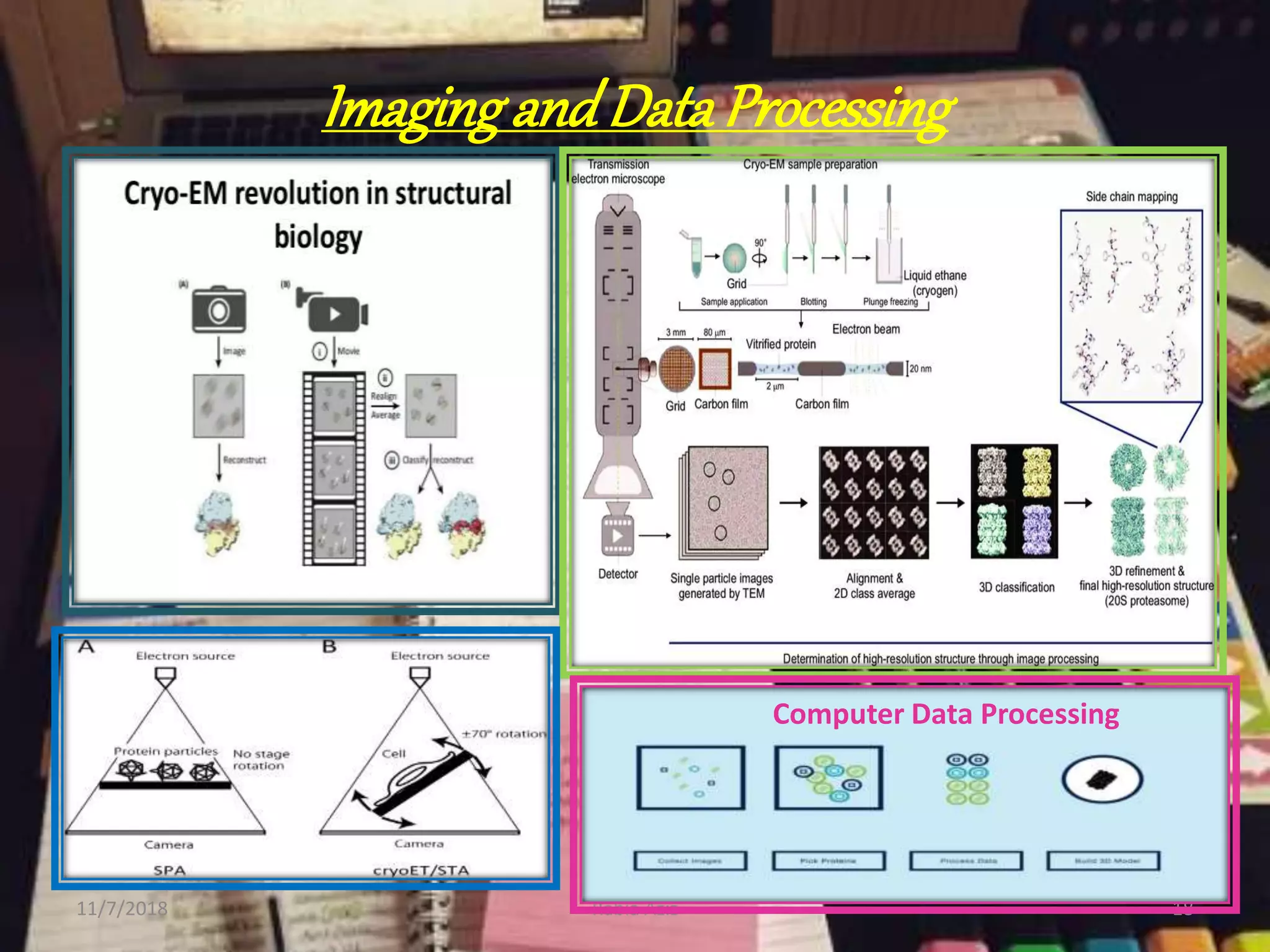
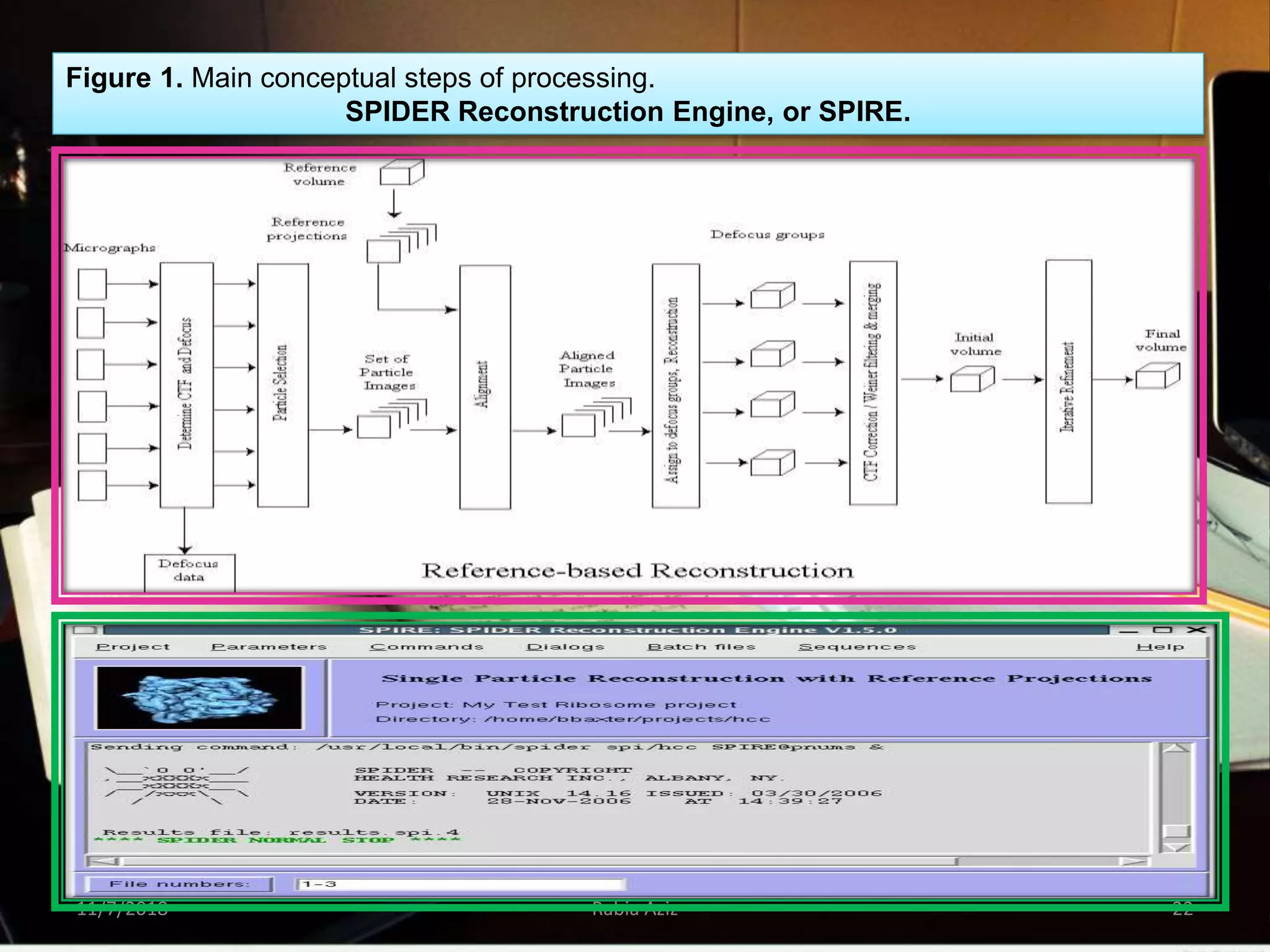
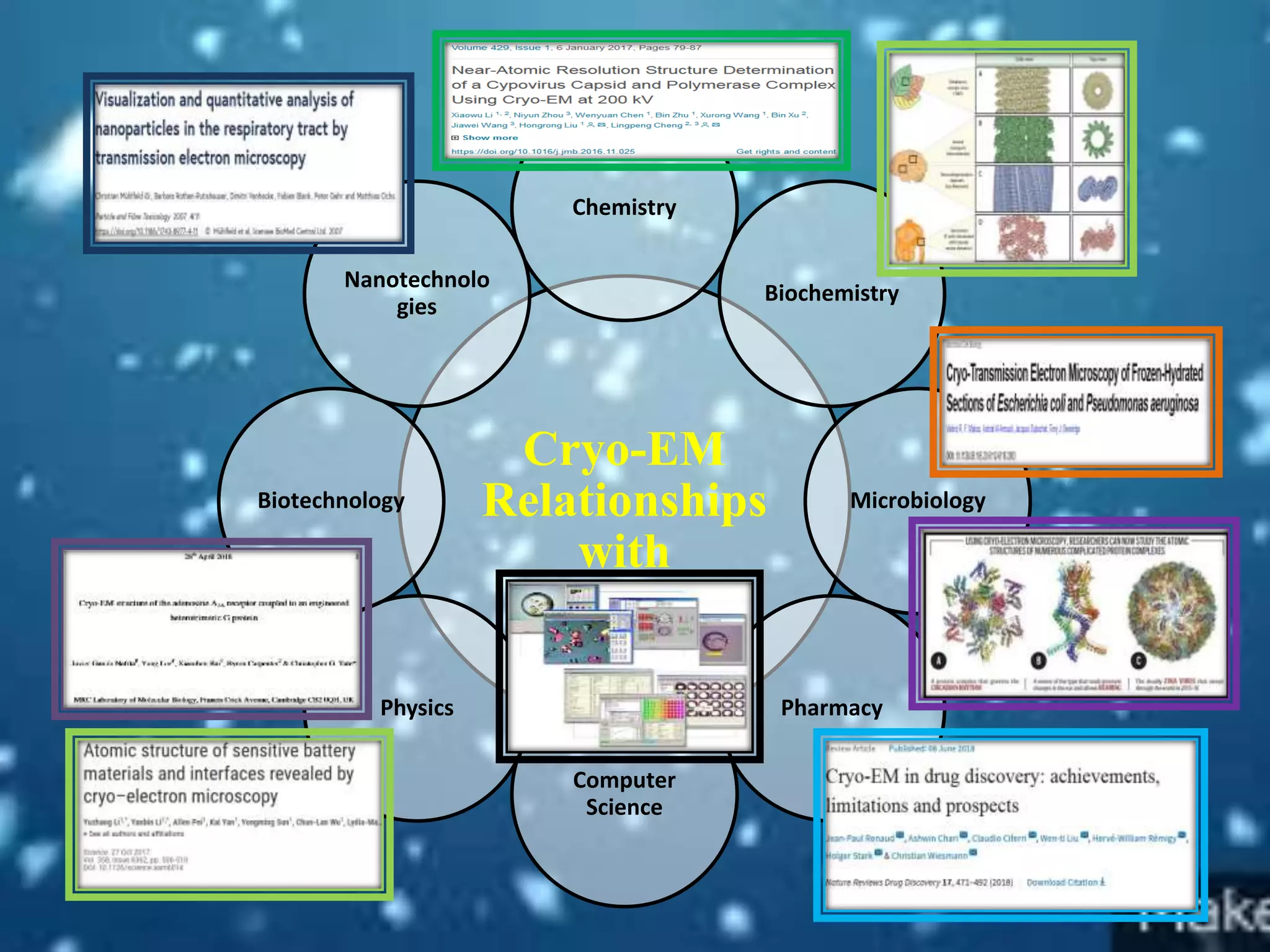
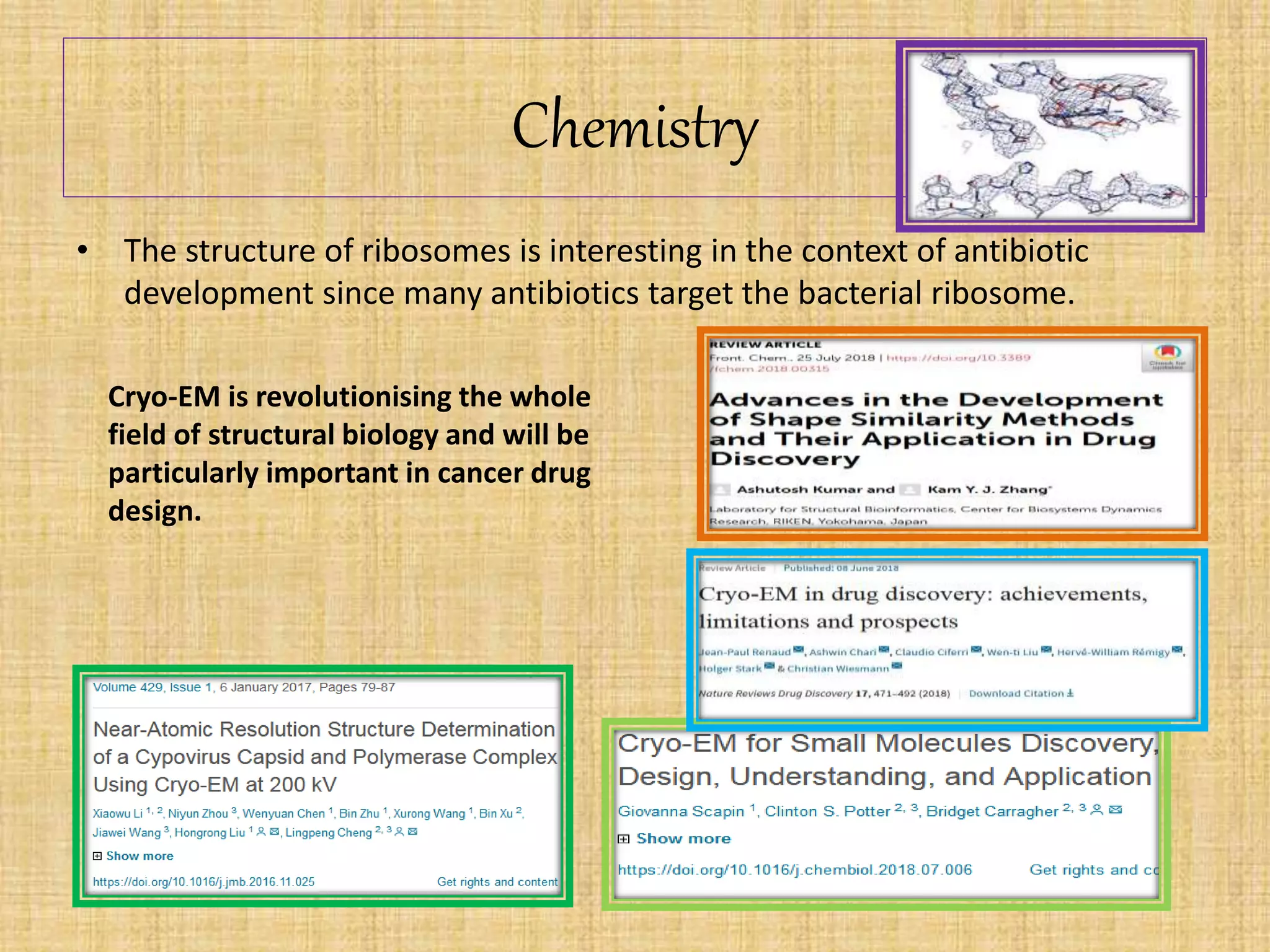
The document discusses the Nobel Prize in Chemistry, particularly the 2017 award to Joachim Frank for his development of cryo-electron microscopy, a technique vital for imaging biomolecules. It outlines the principles of cryo-electron microscopy, its applications in structural biology, and the methodology involved in preparing samples for imaging. Additionally, it highlights the advantages and future prospects of cryo-em technology in various scientific fields, including pharmacology and drug design.