Embed presentation
Downloaded 128 times

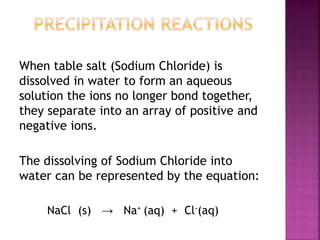



When sodium chloride dissolves in water, the ions separate from each other into positive sodium ions and negative chloride ions. This dissolving process can be represented by the equation NaCl(s) → Na+(aq) + Cl-(aq). The solubility of compounds is based on rules regarding which ions combine to form soluble or insoluble compounds. Compounds that do not dissolve in water and remain as a solid are considered precipitates, and chemical reactions where precipitates form are called precipitation reactions.




