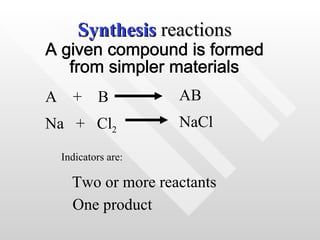
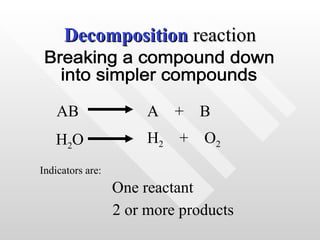
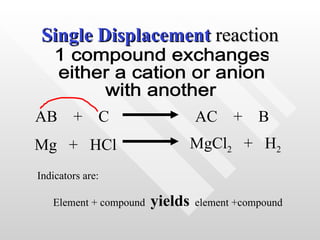
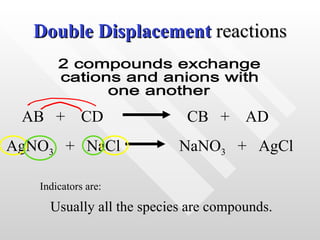
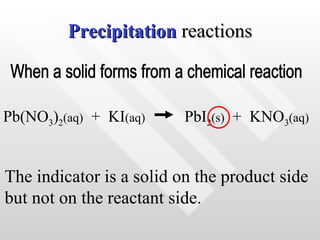
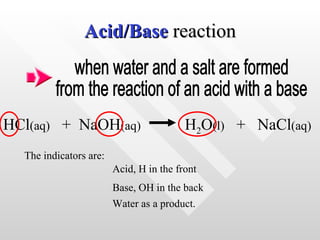
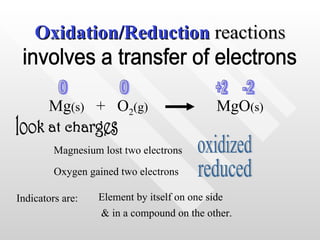
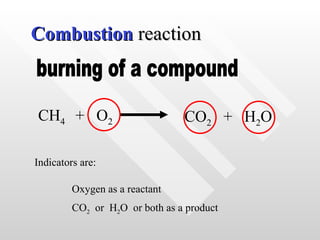
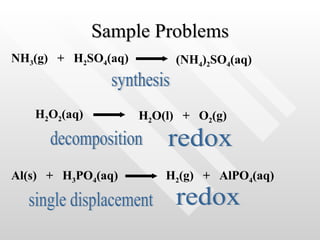
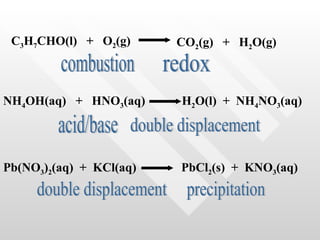
The document classifies different types of chemical reactions:
1) Synthesis reactions involve combining two or more reactants to form one product.
2) Decomposition reactions involve breaking down one reactant into two or more products.
3) Single displacement reactions involve one compound exchanging ions with another.
4) Double displacement reactions involve two compounds exchanging ions to form two new compounds.