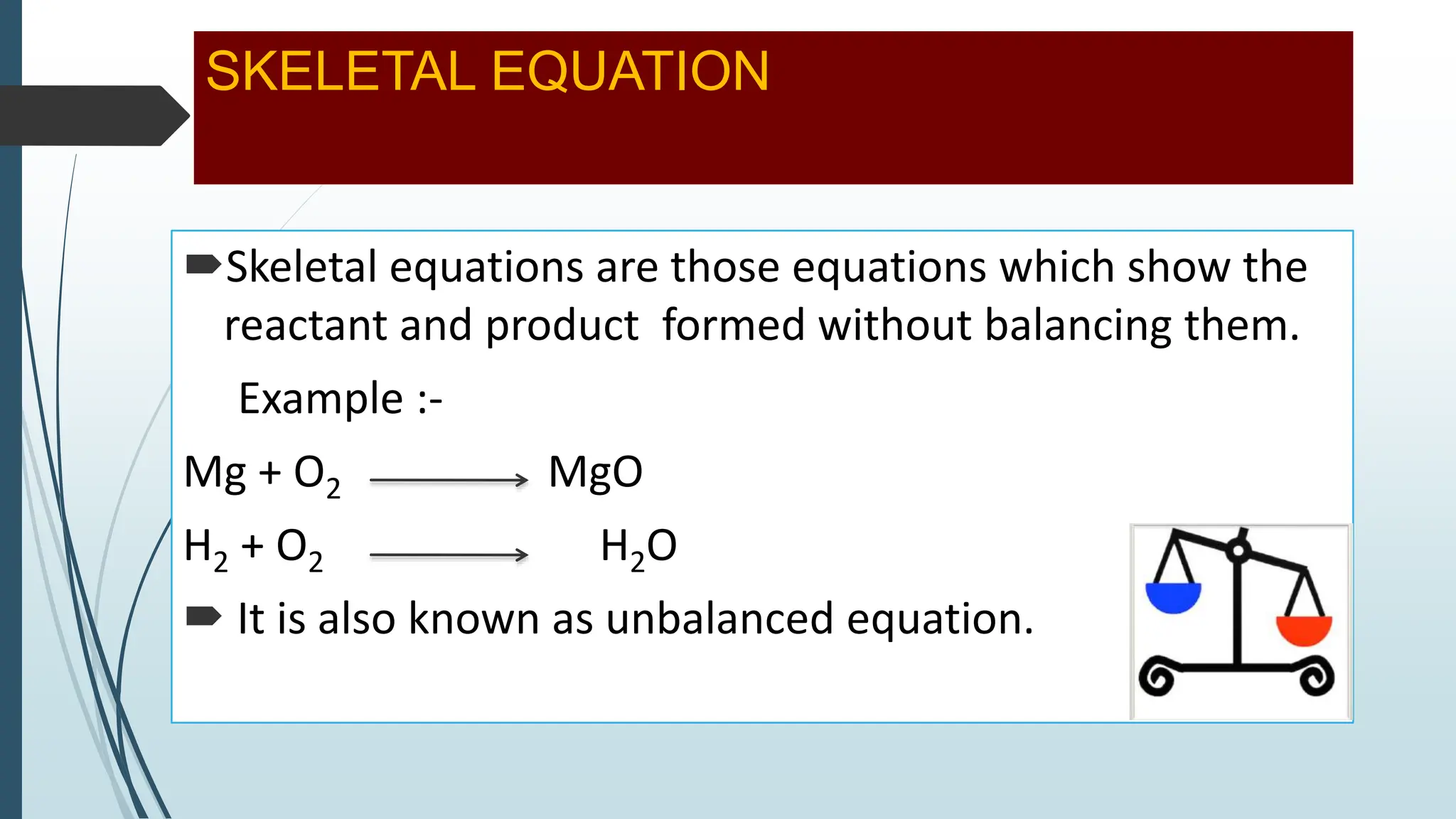
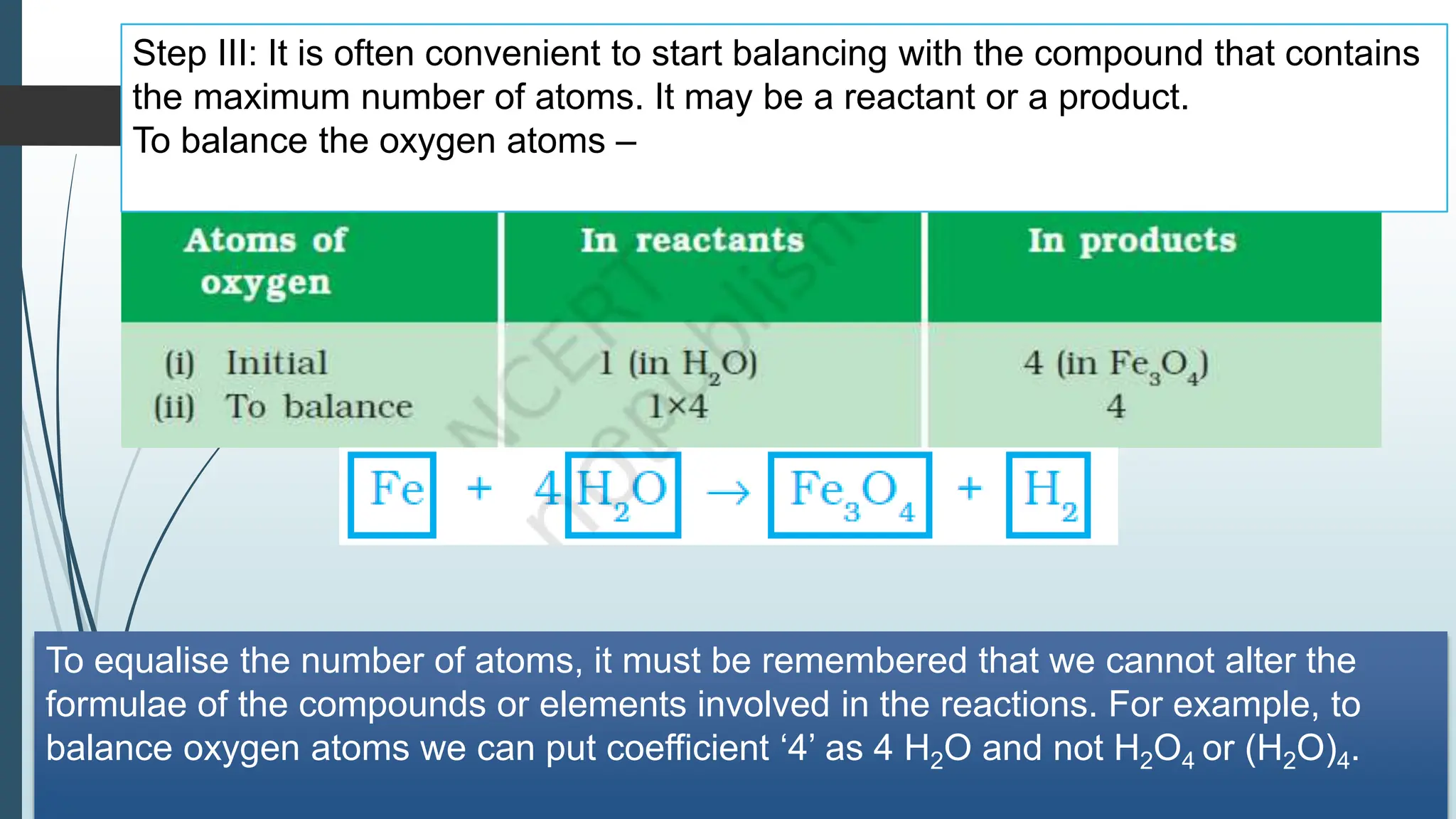
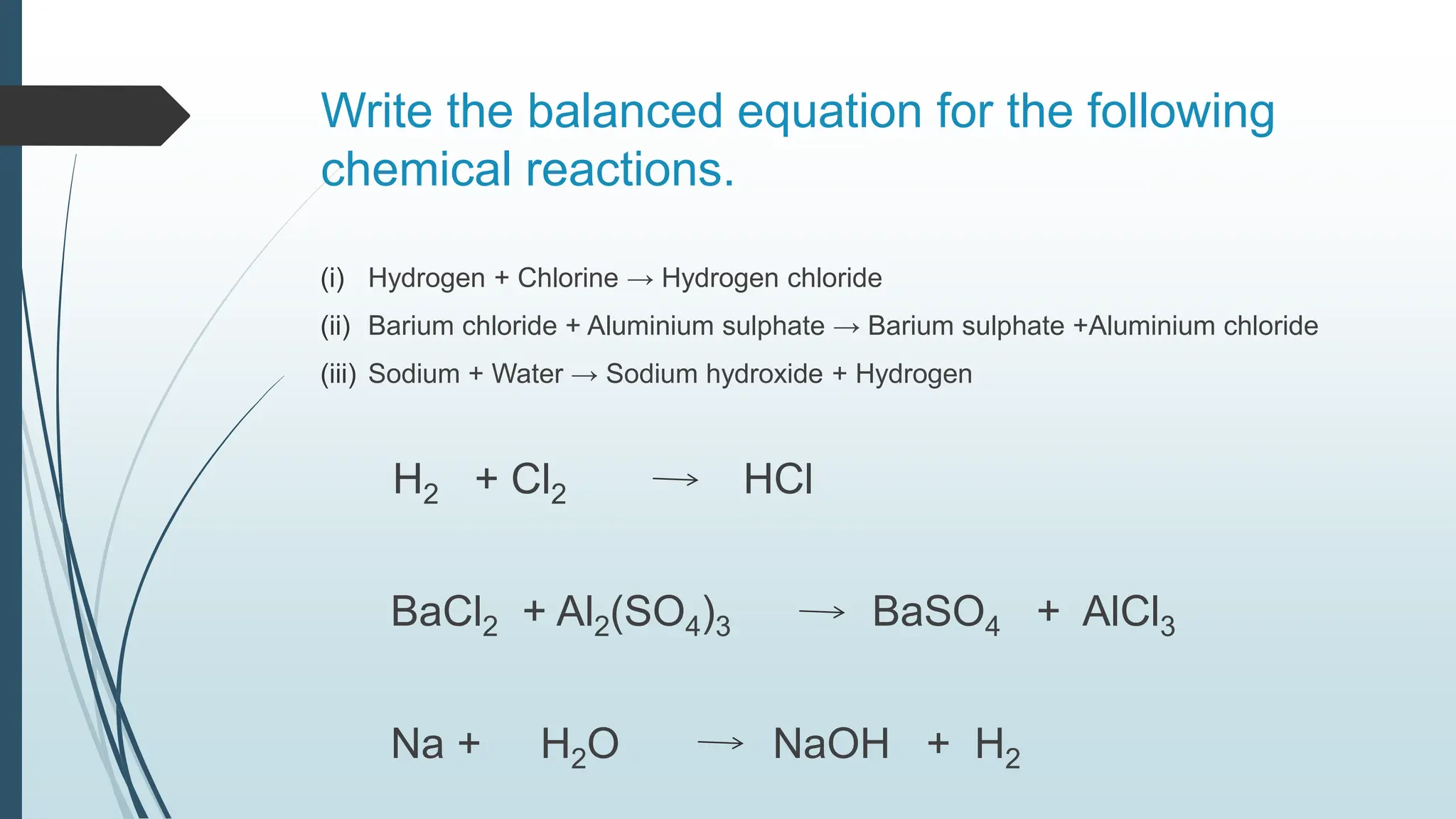
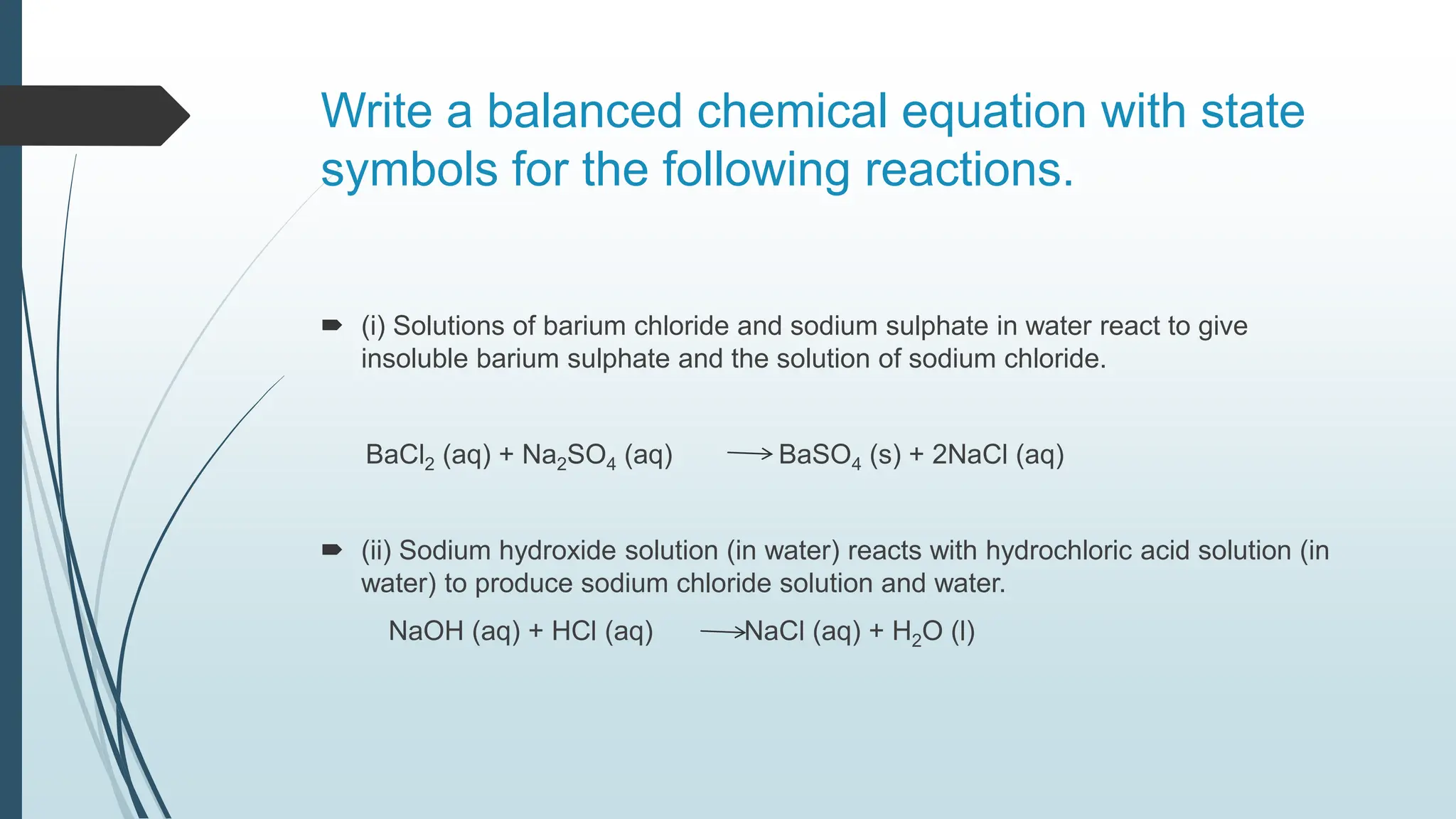
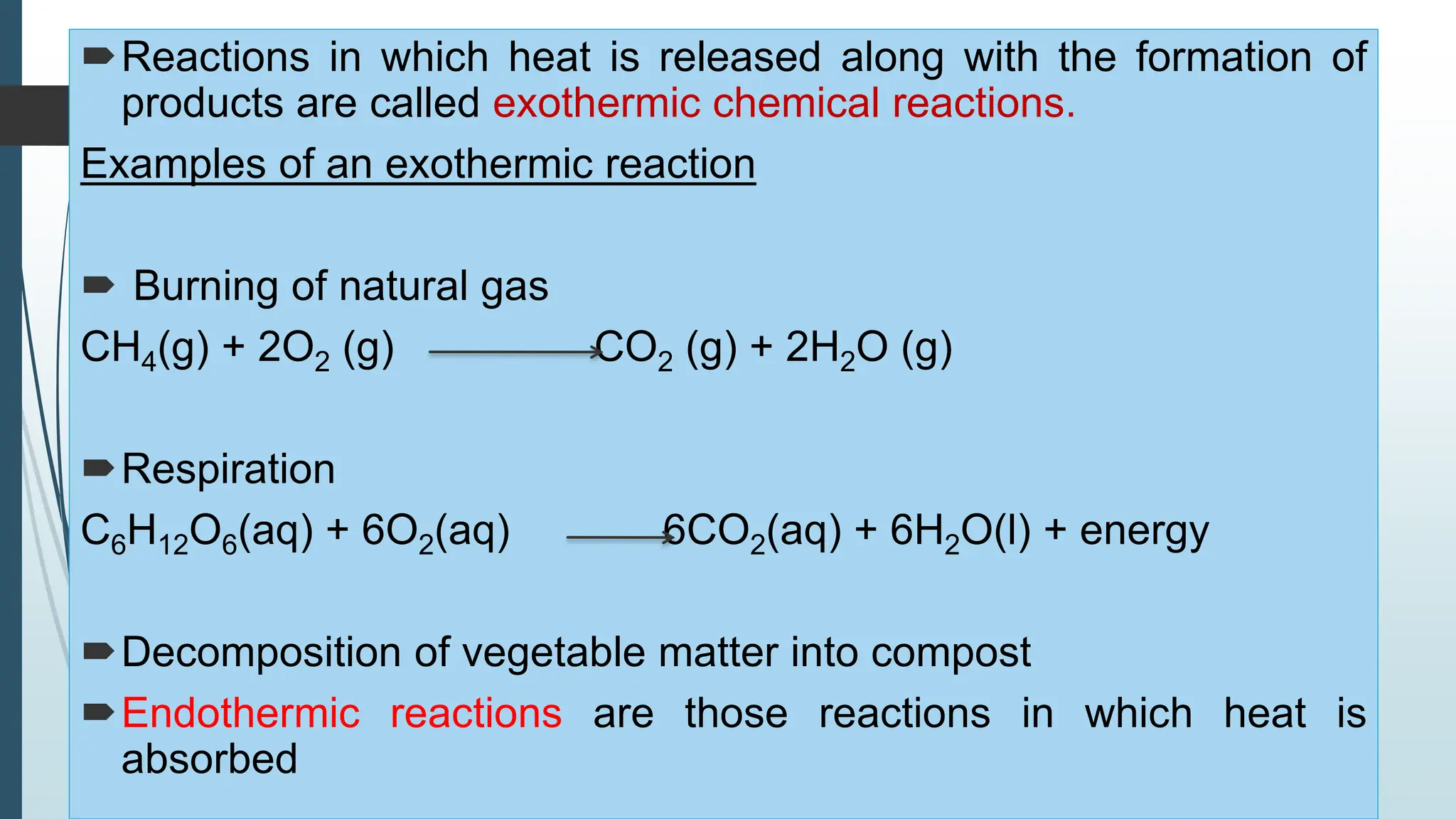
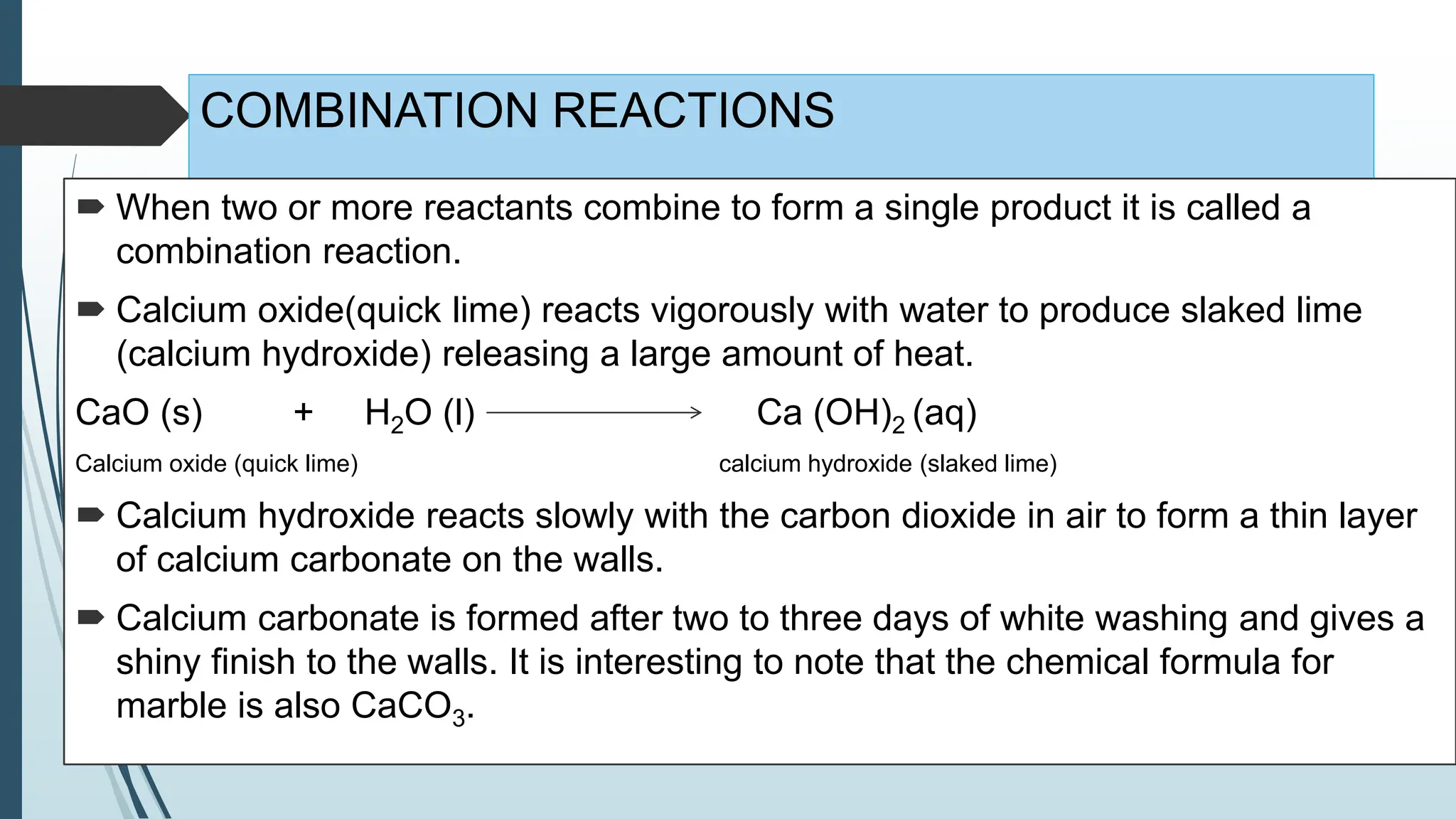
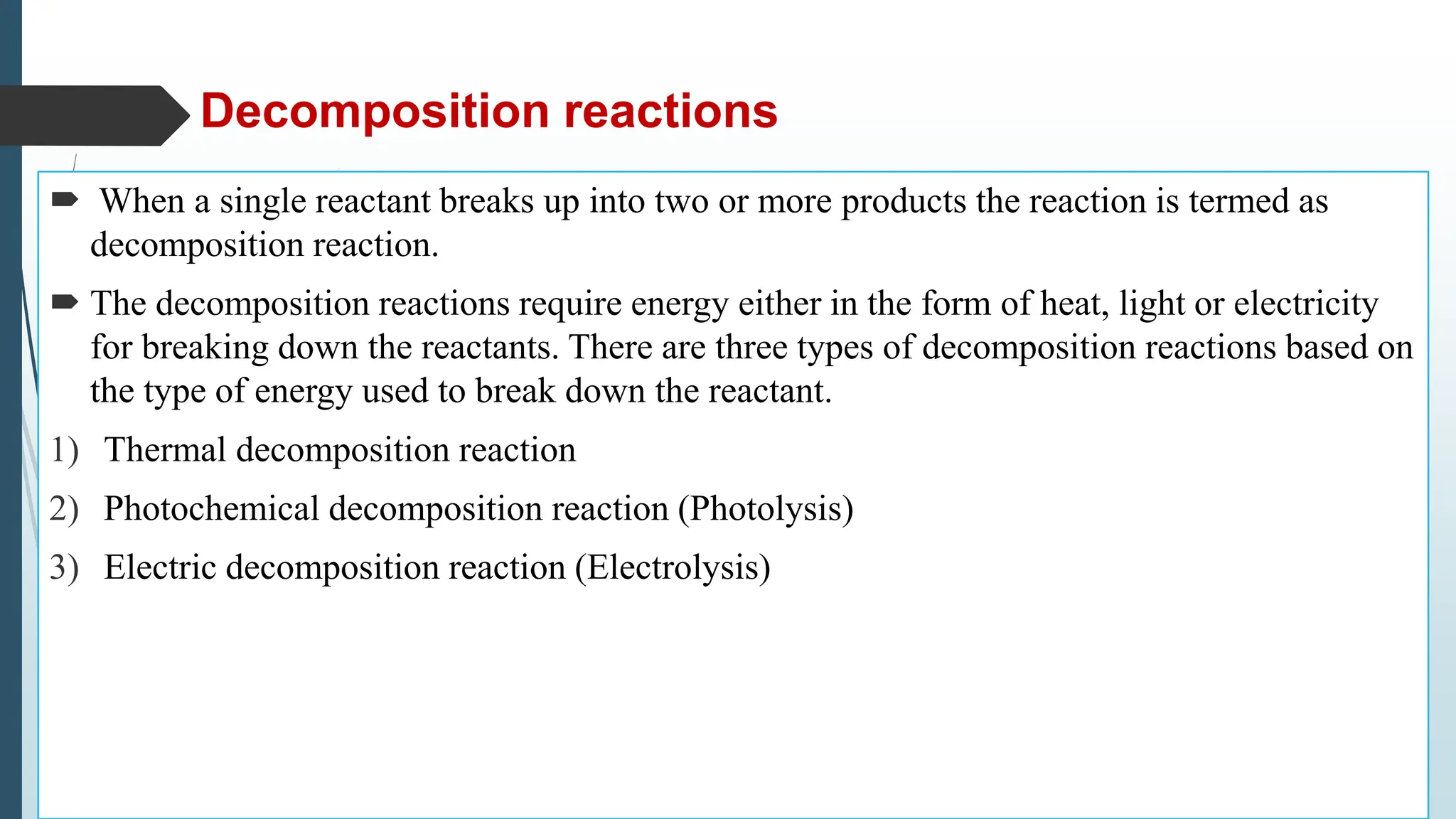
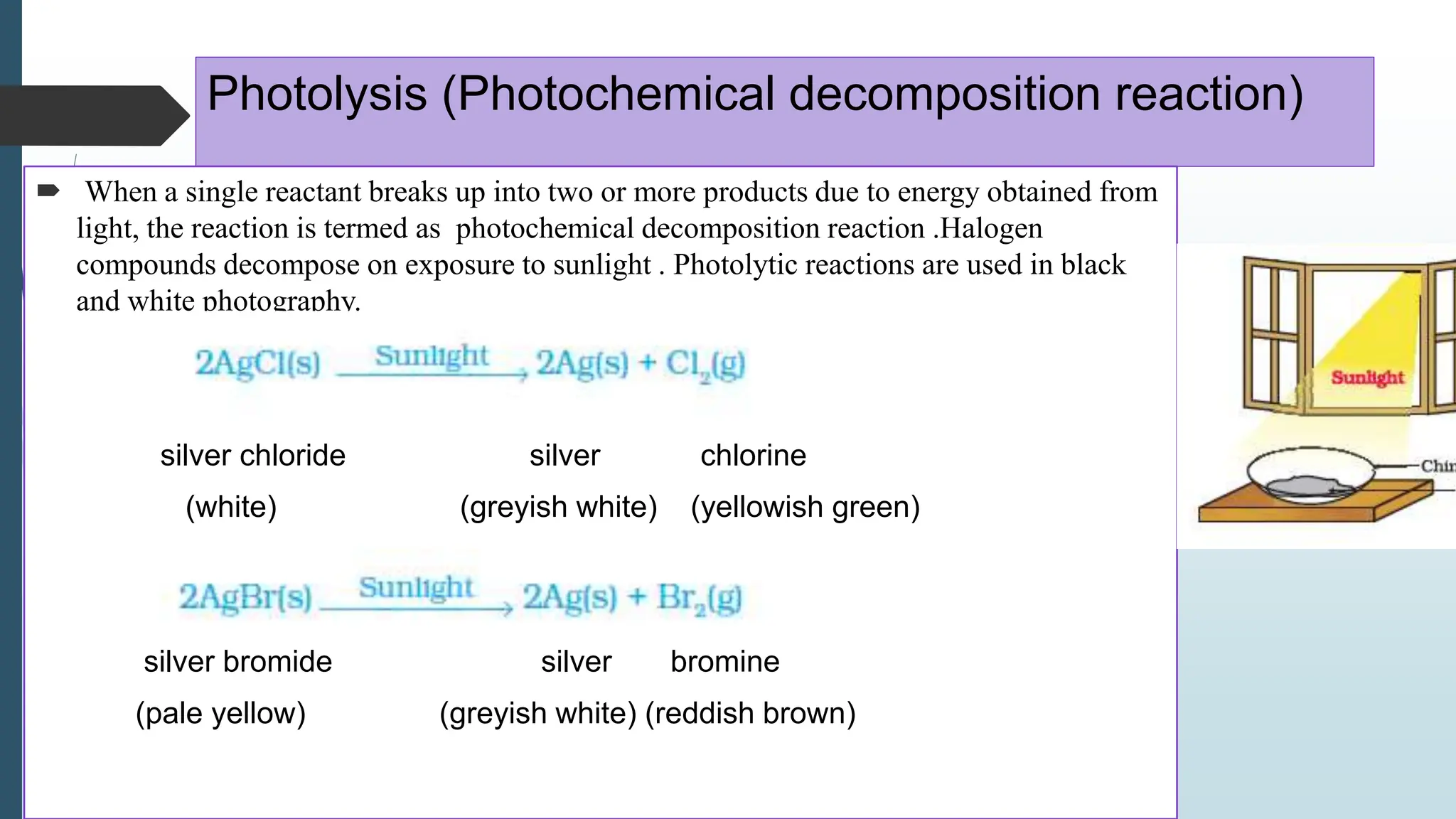
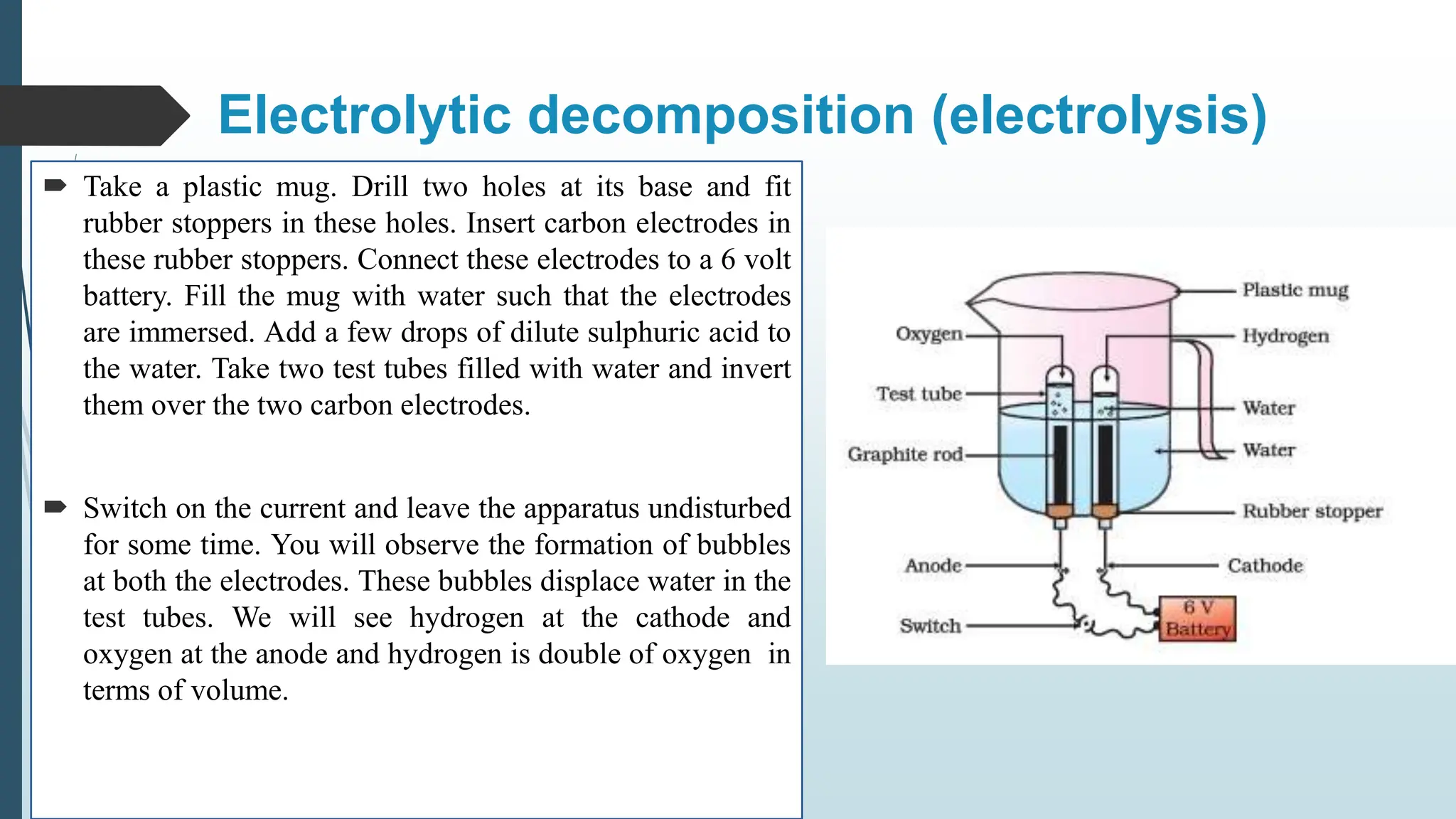
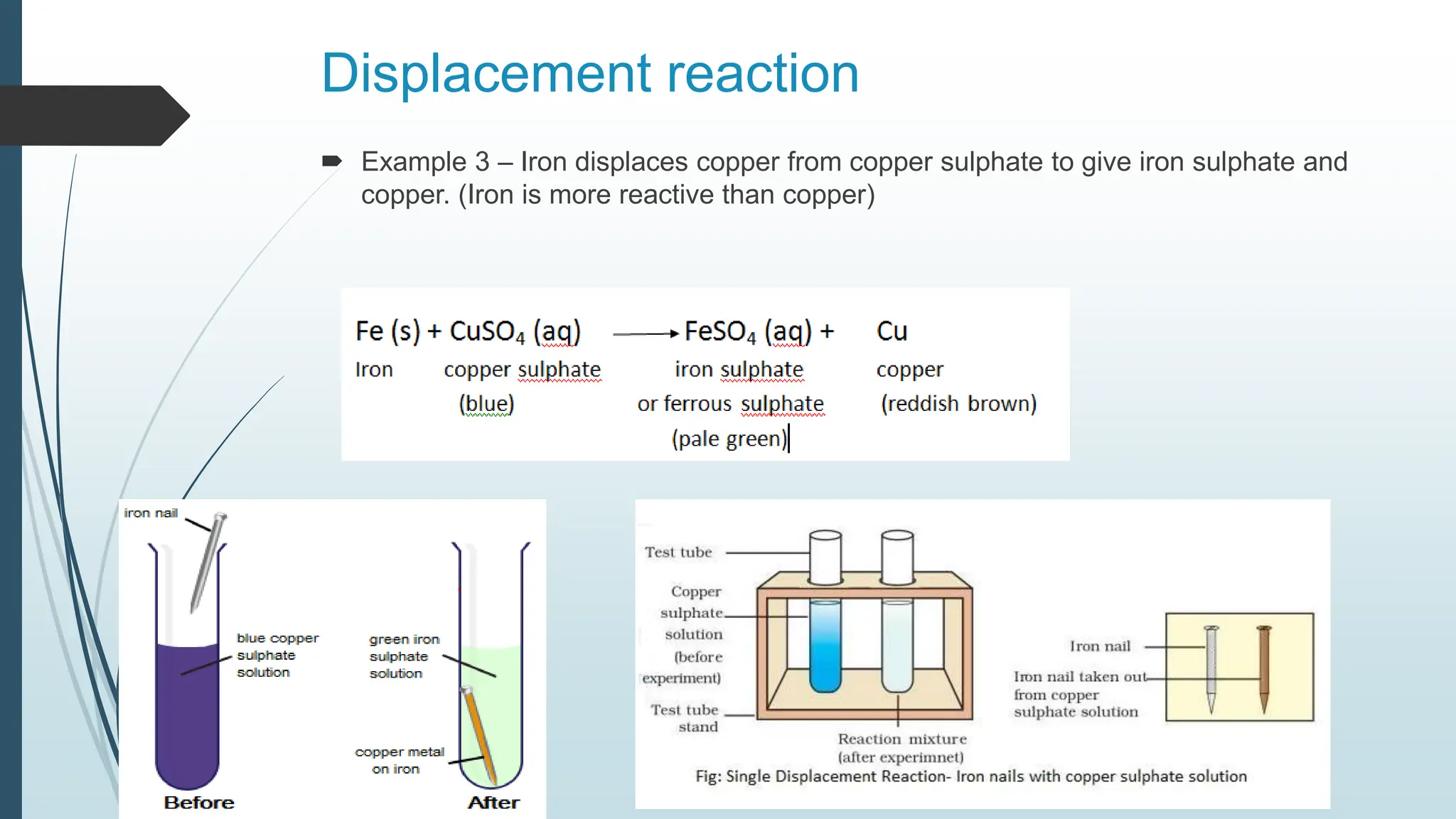
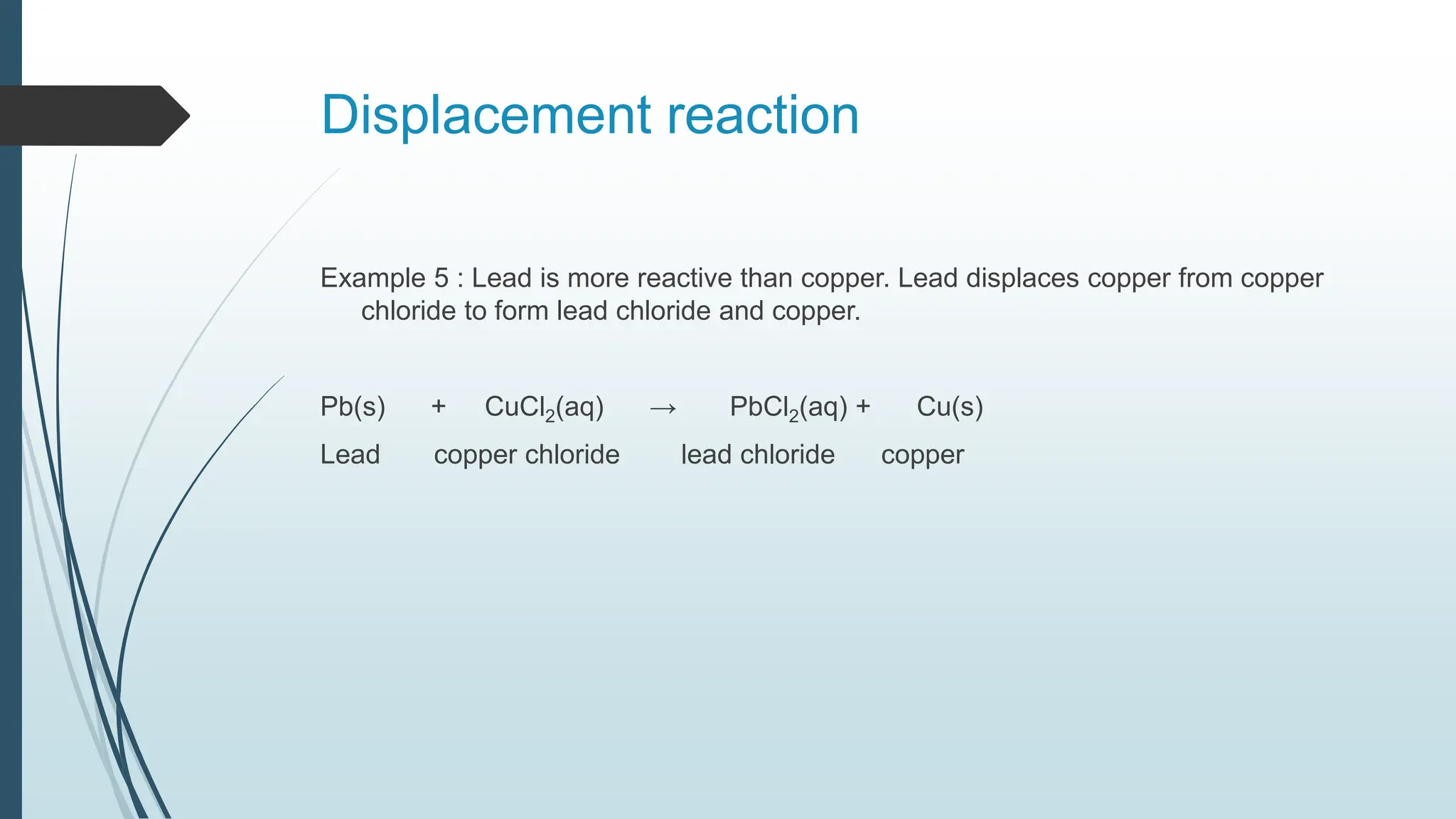
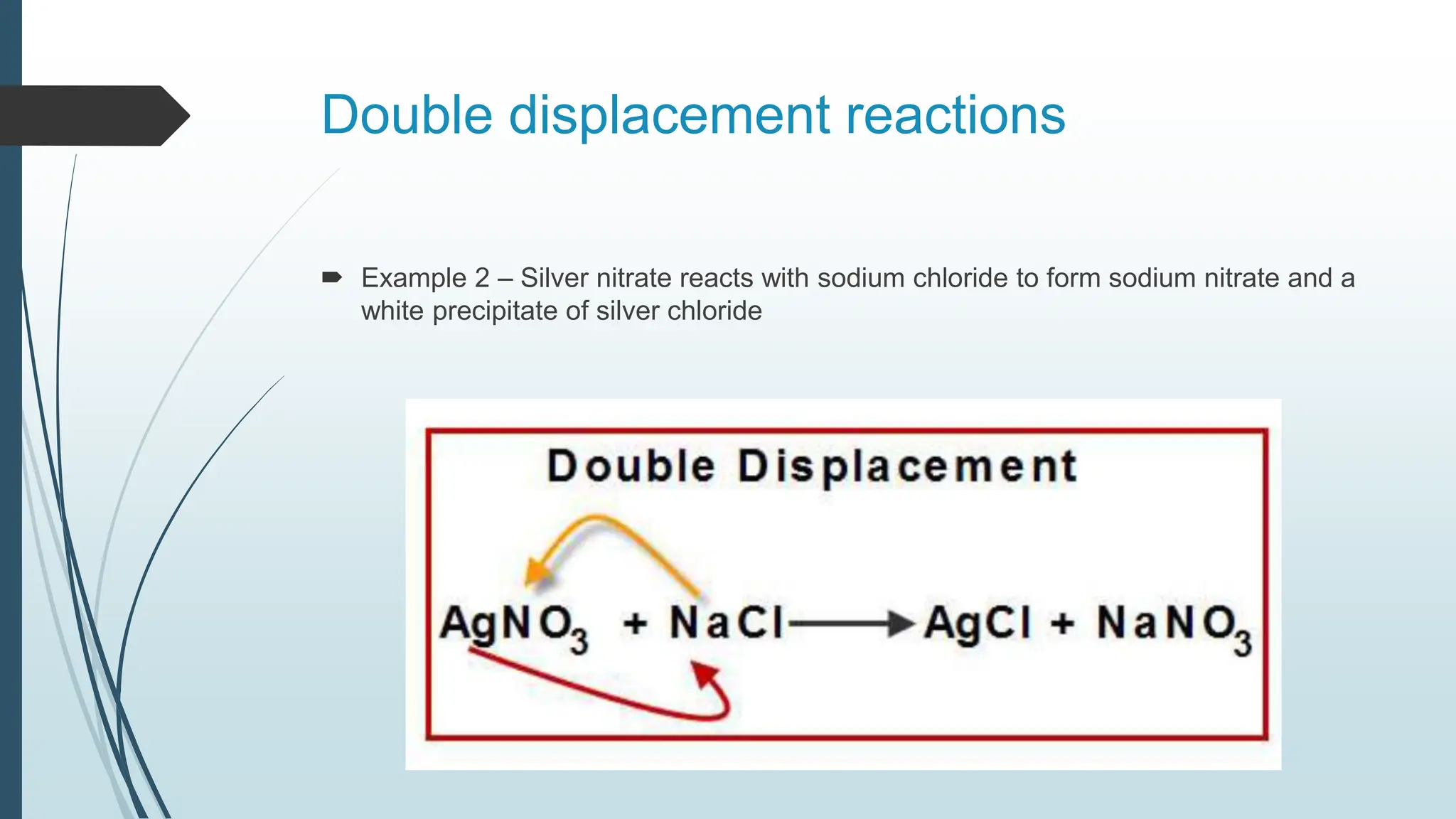
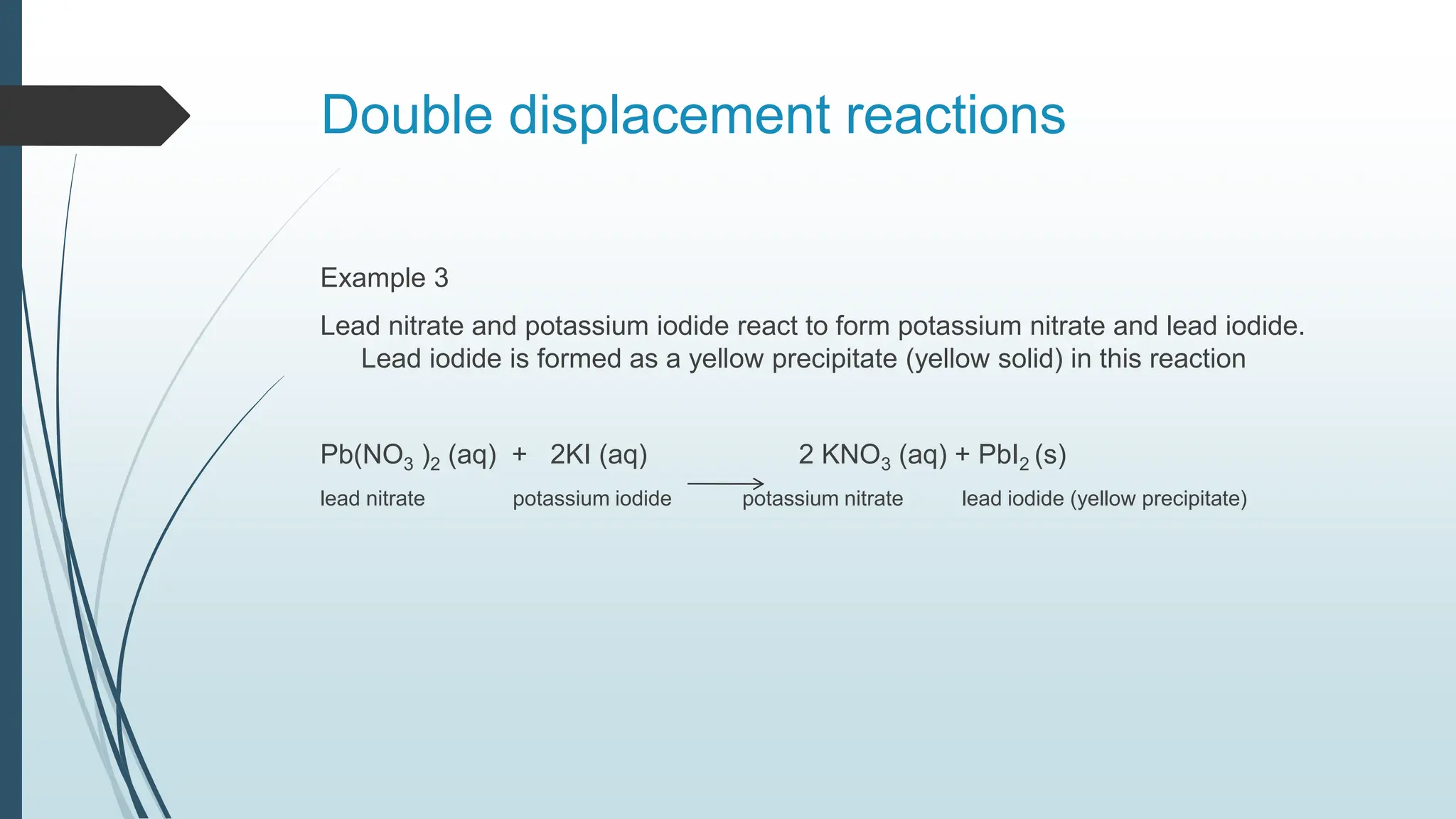
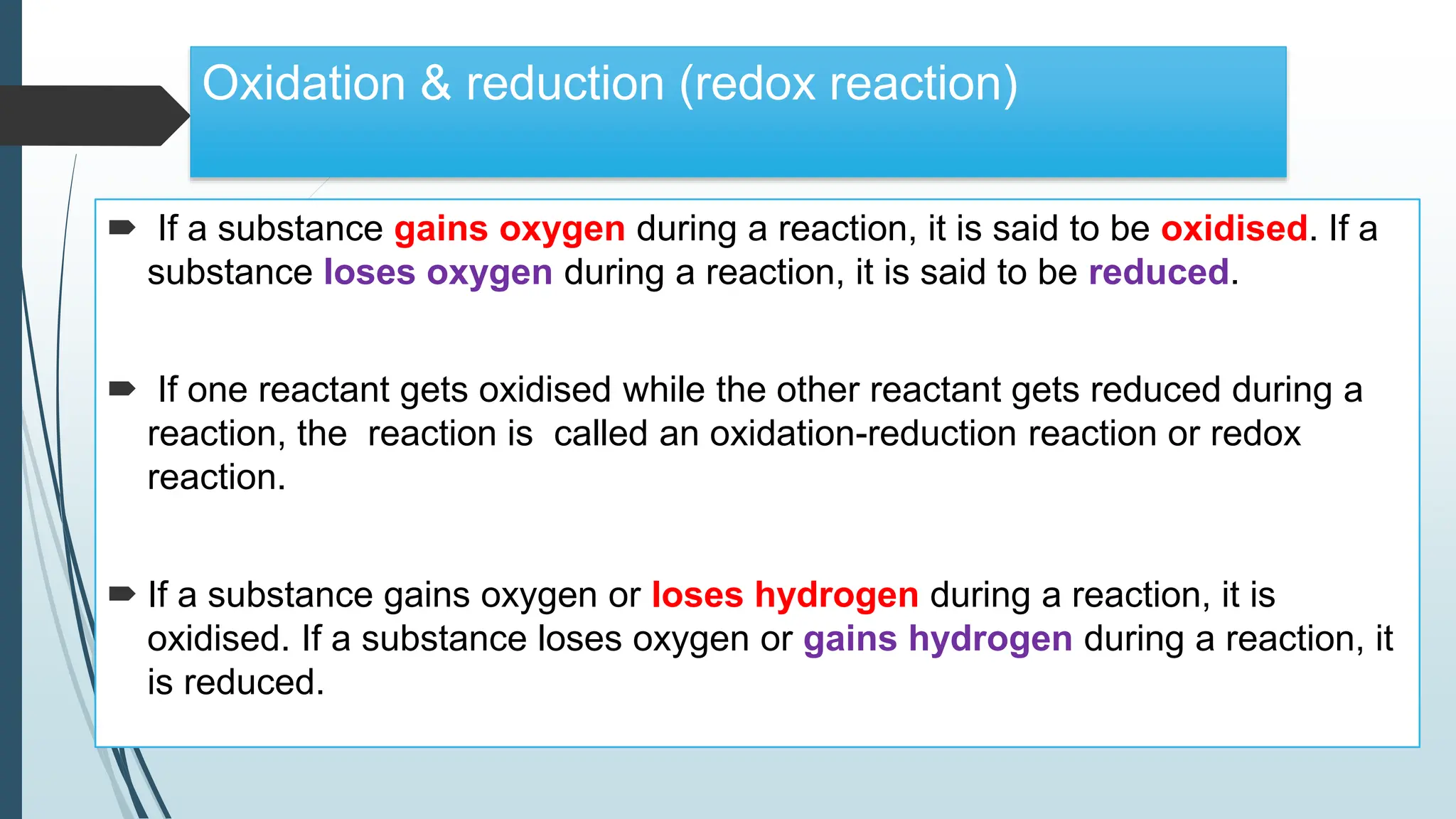
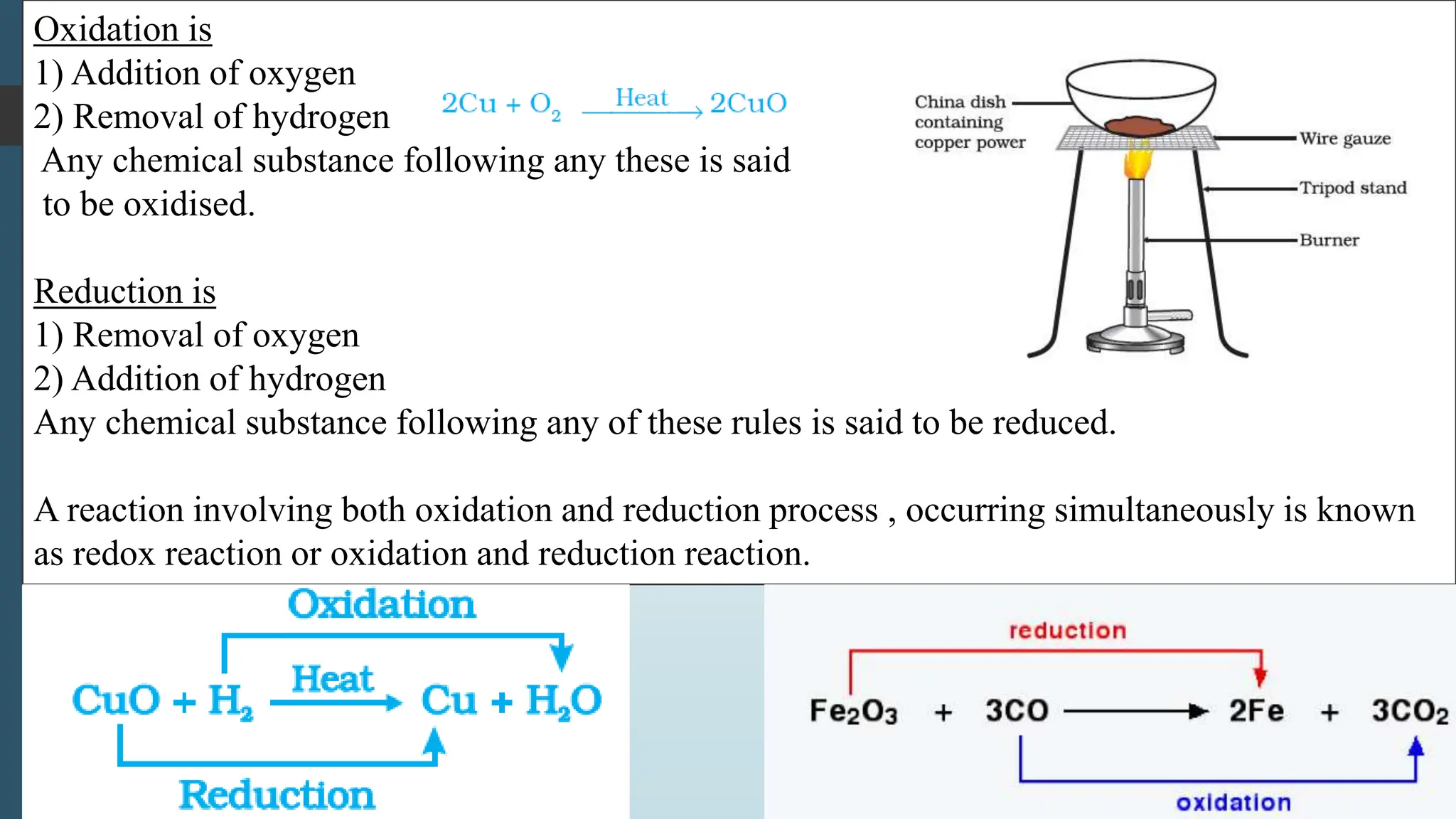
The document discusses chemical reactions and equations, defining chemical reactions as transformations of substances that can be represented symbolically through equations. It explains various types of reactions, including combination, decomposition, displacement, double displacement, and redox reactions, while highlighting the need for balancing chemical equations to adhere to the conservation of mass. The document also touches on practical examples, the effects of reactions such as corrosion and rancidity, and methods of preventing deterioration of materials.