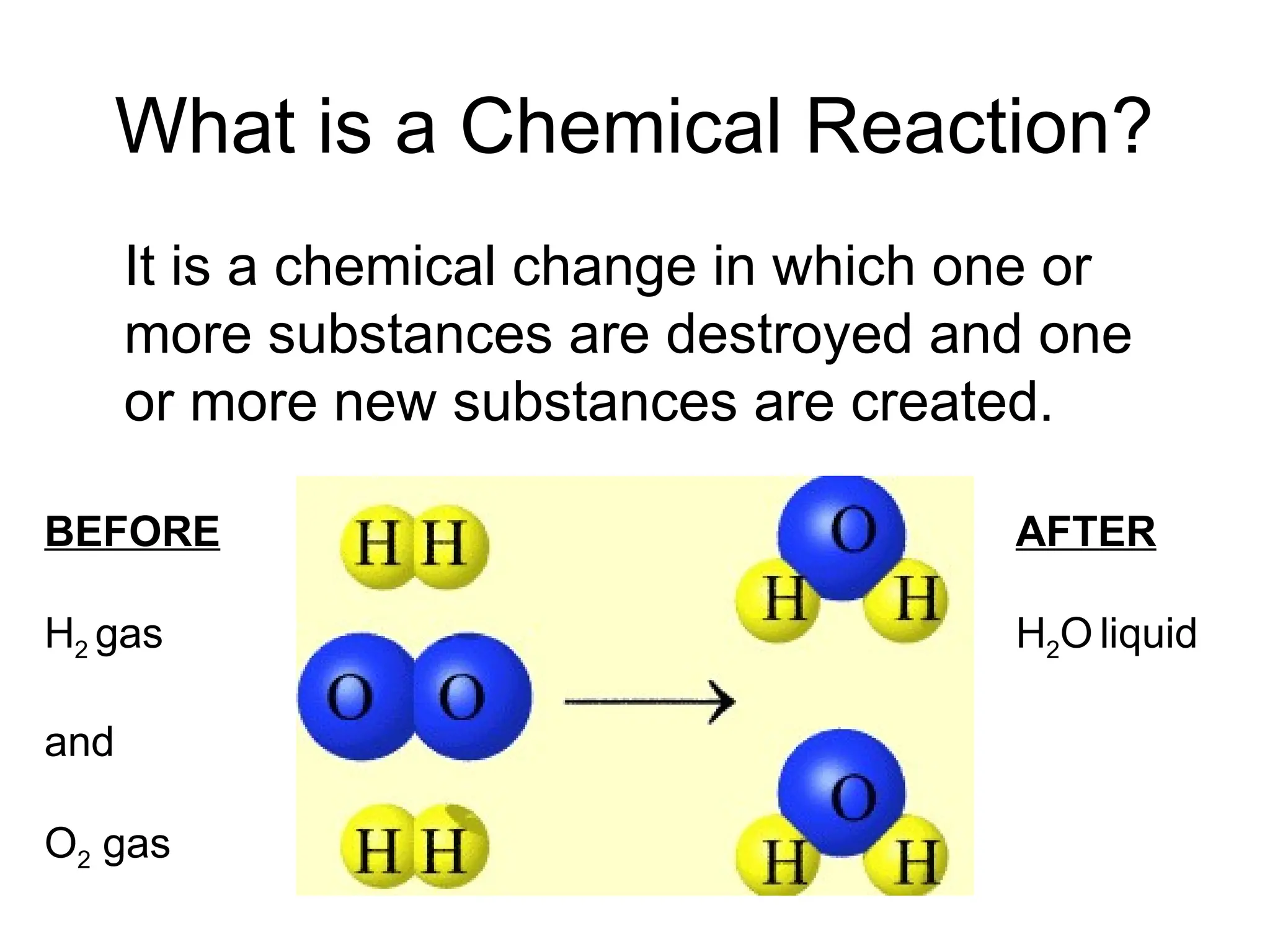
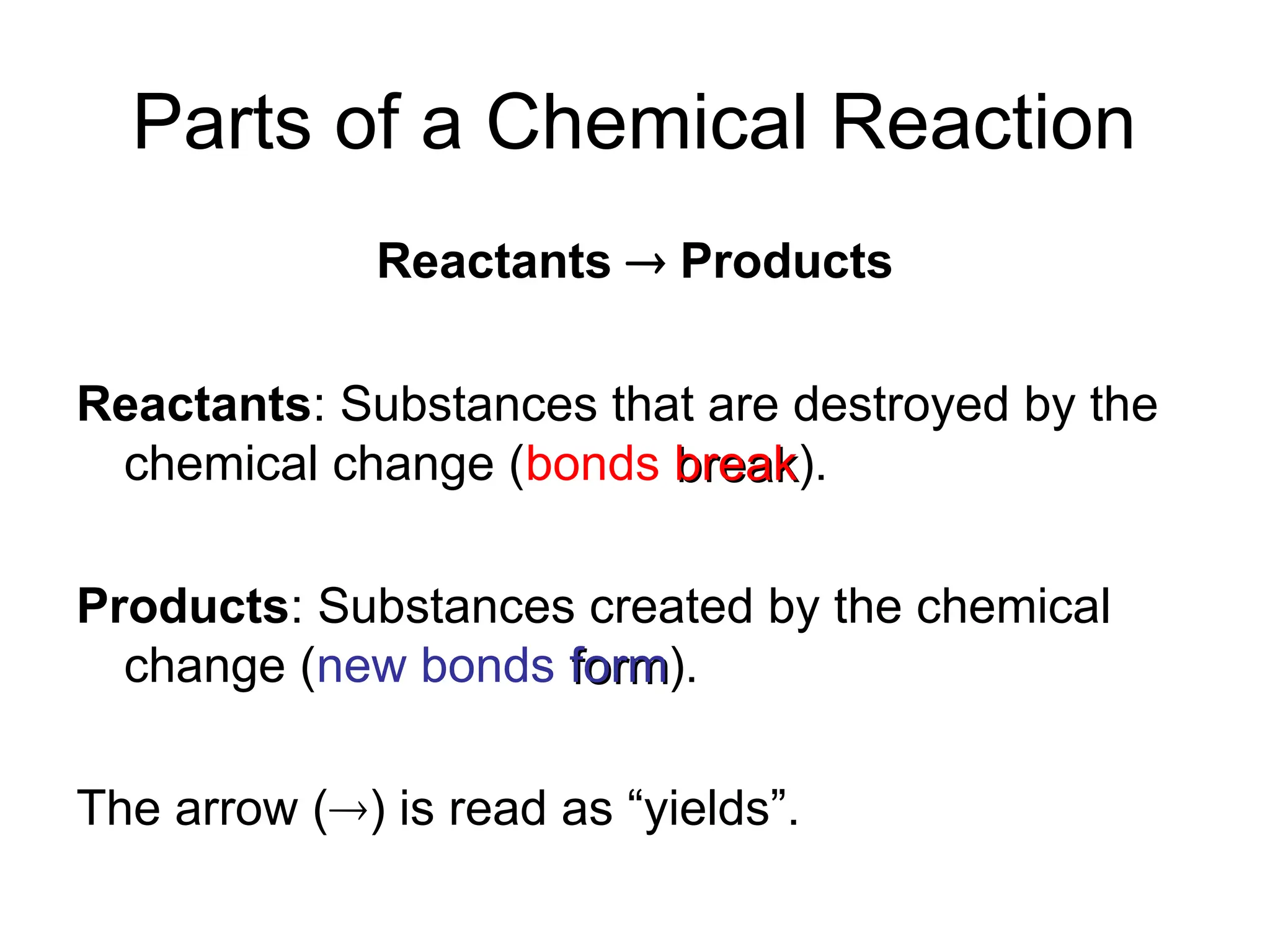
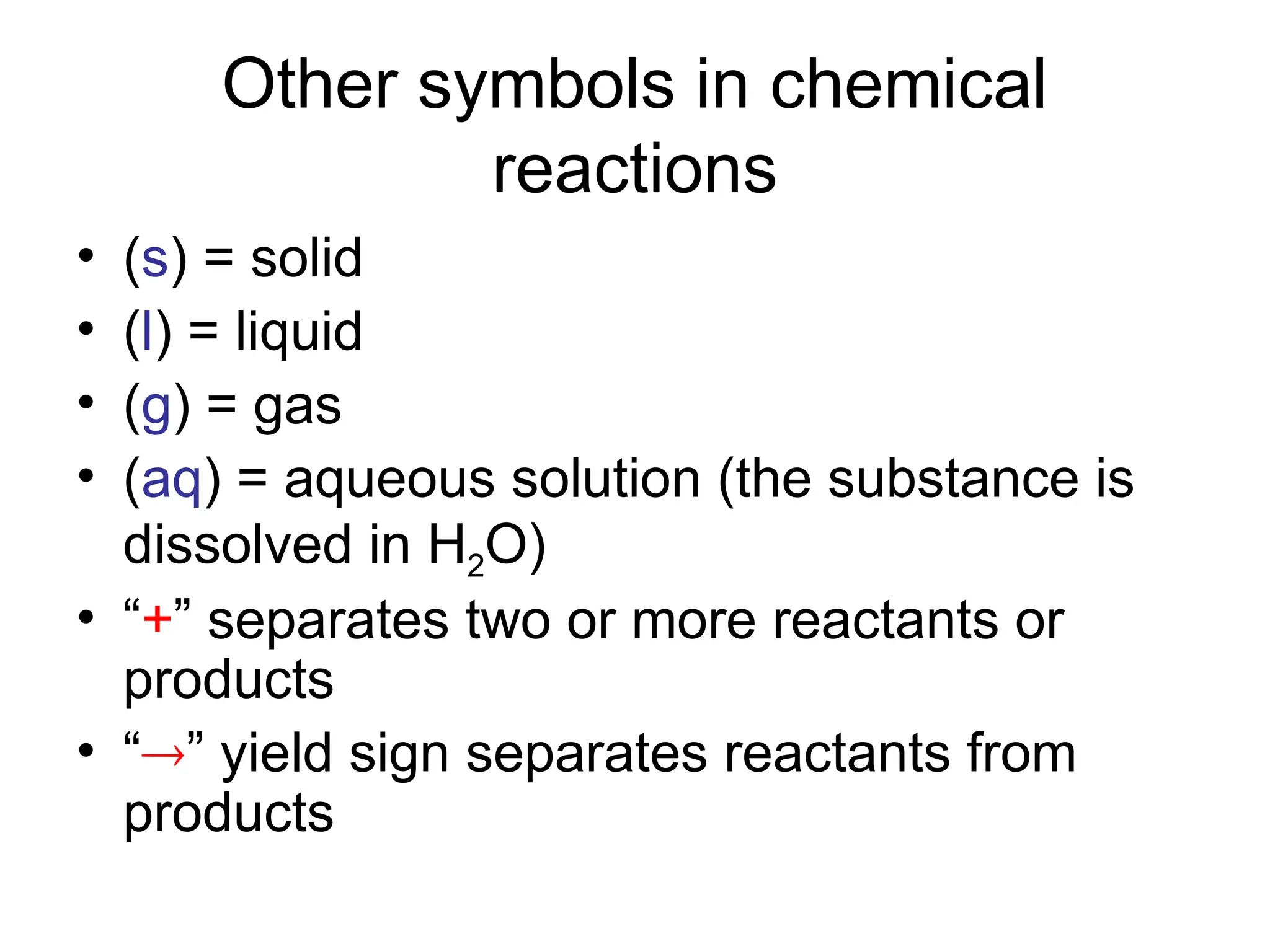
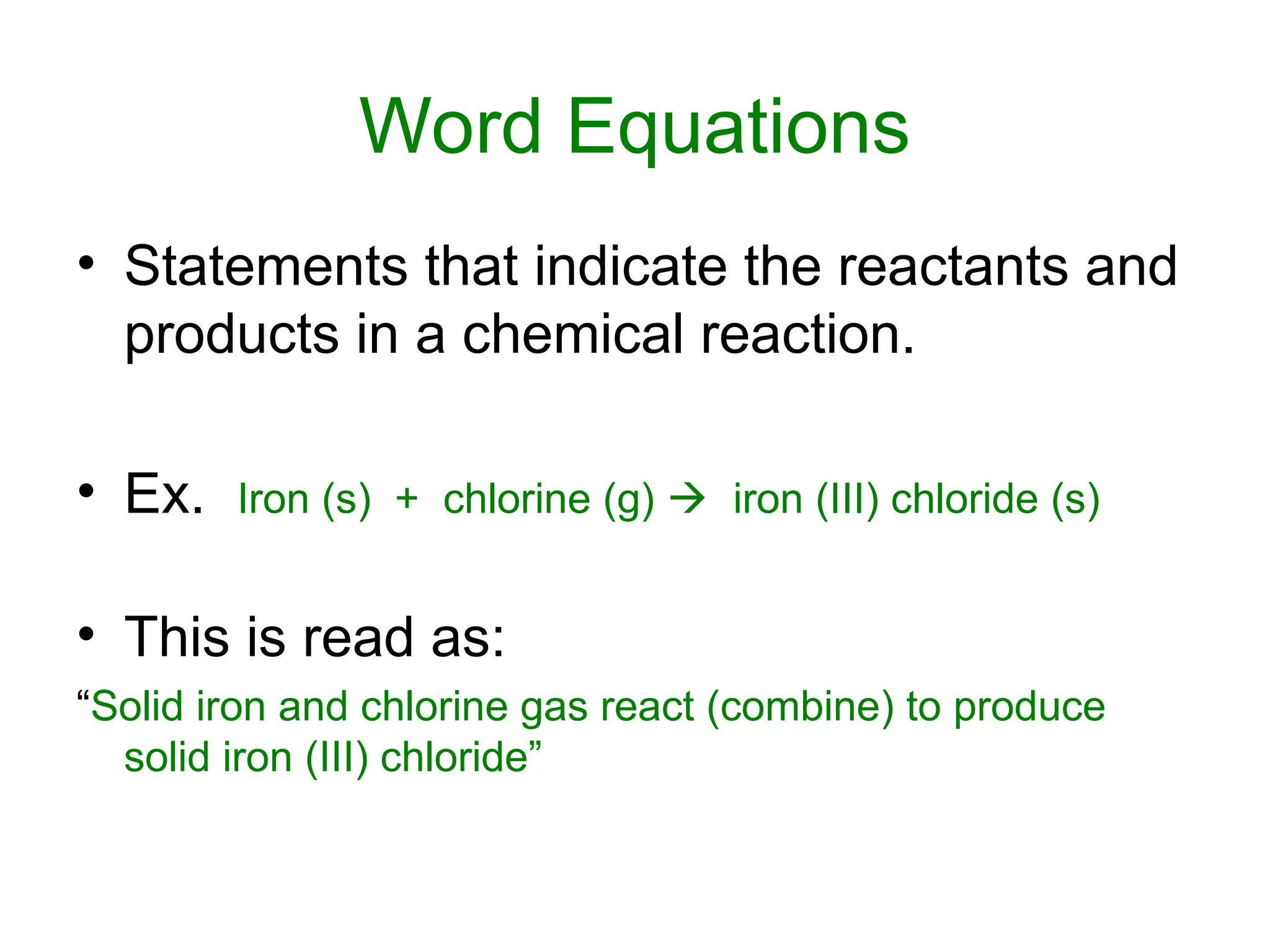
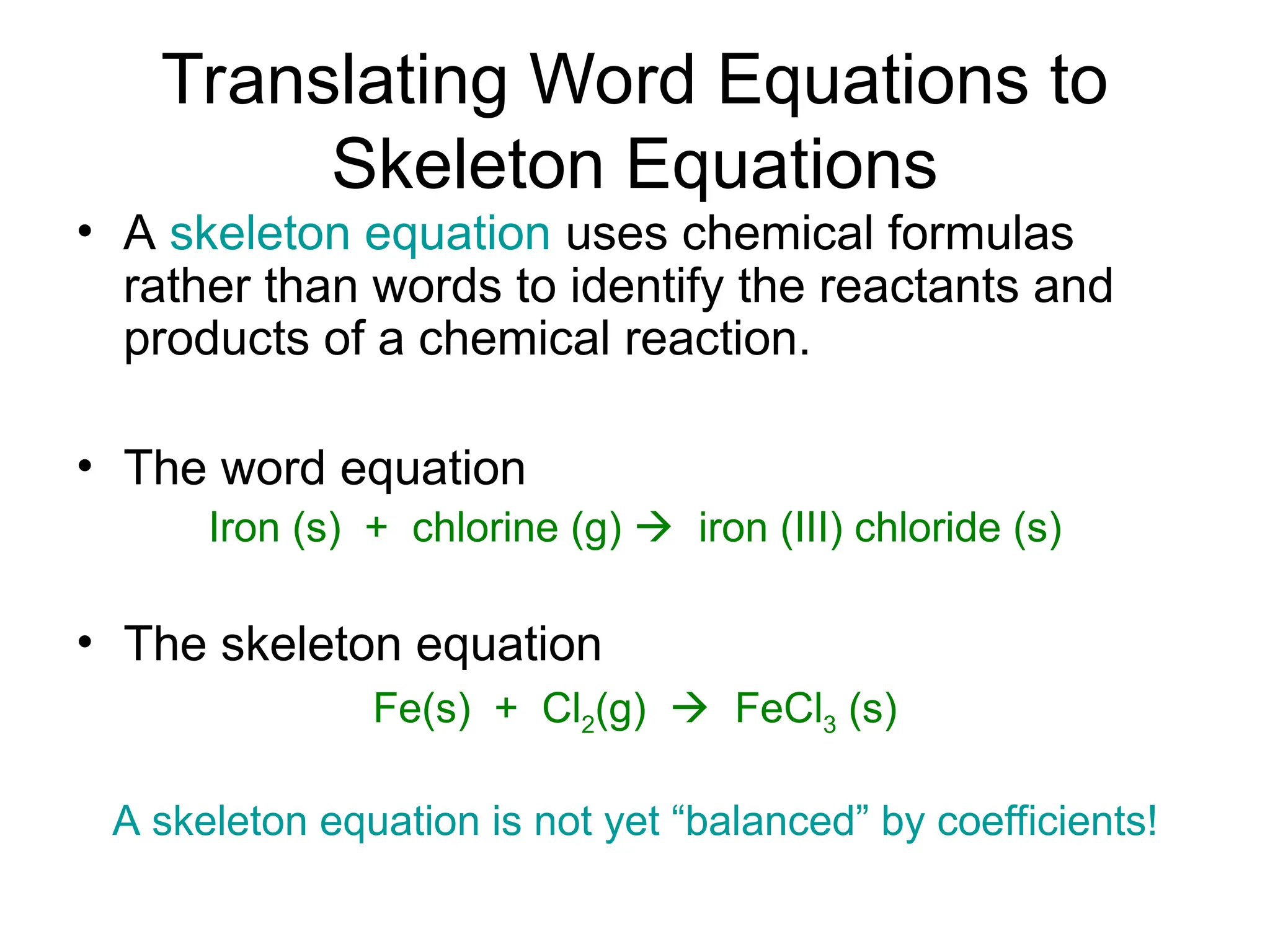
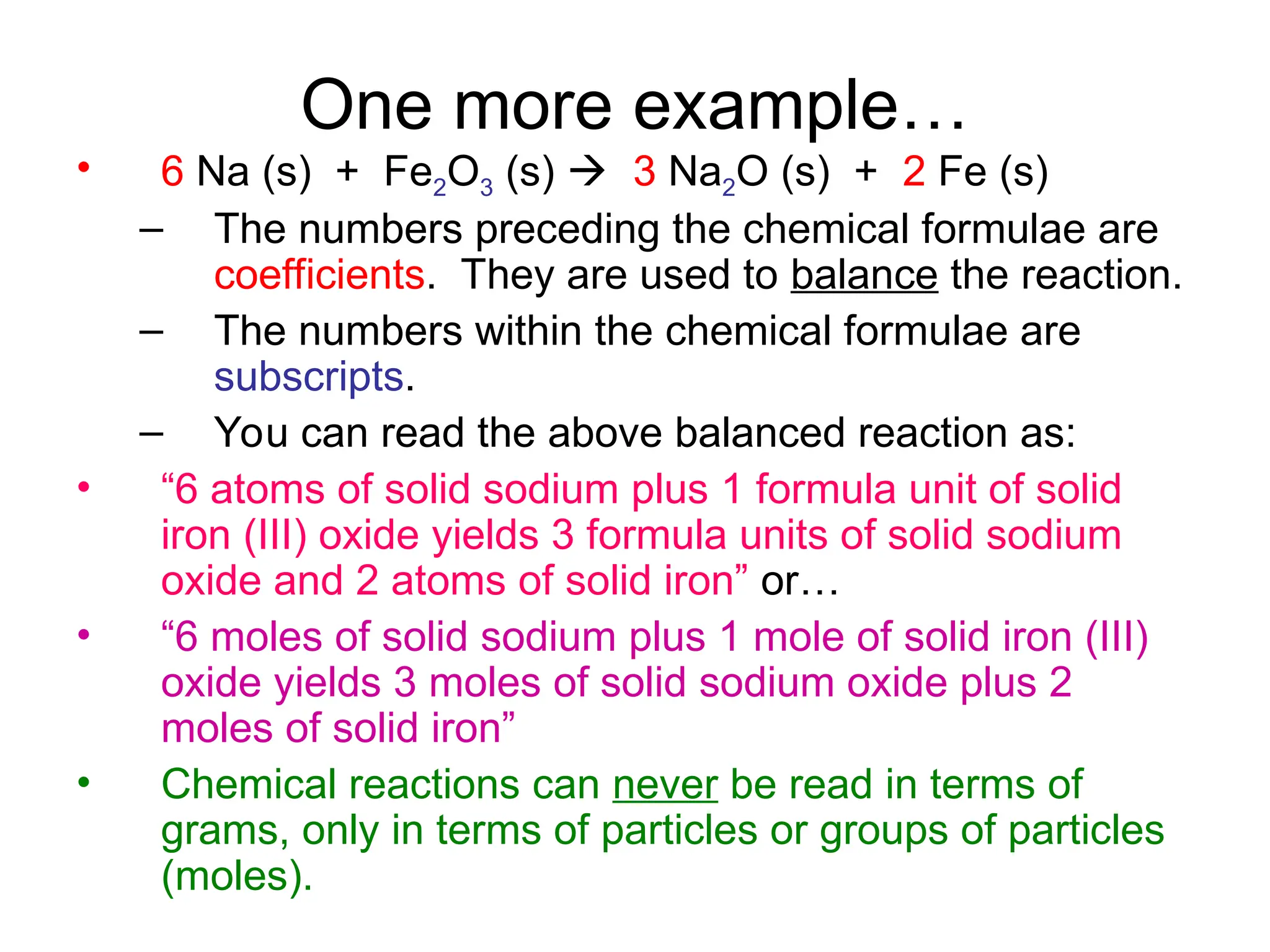
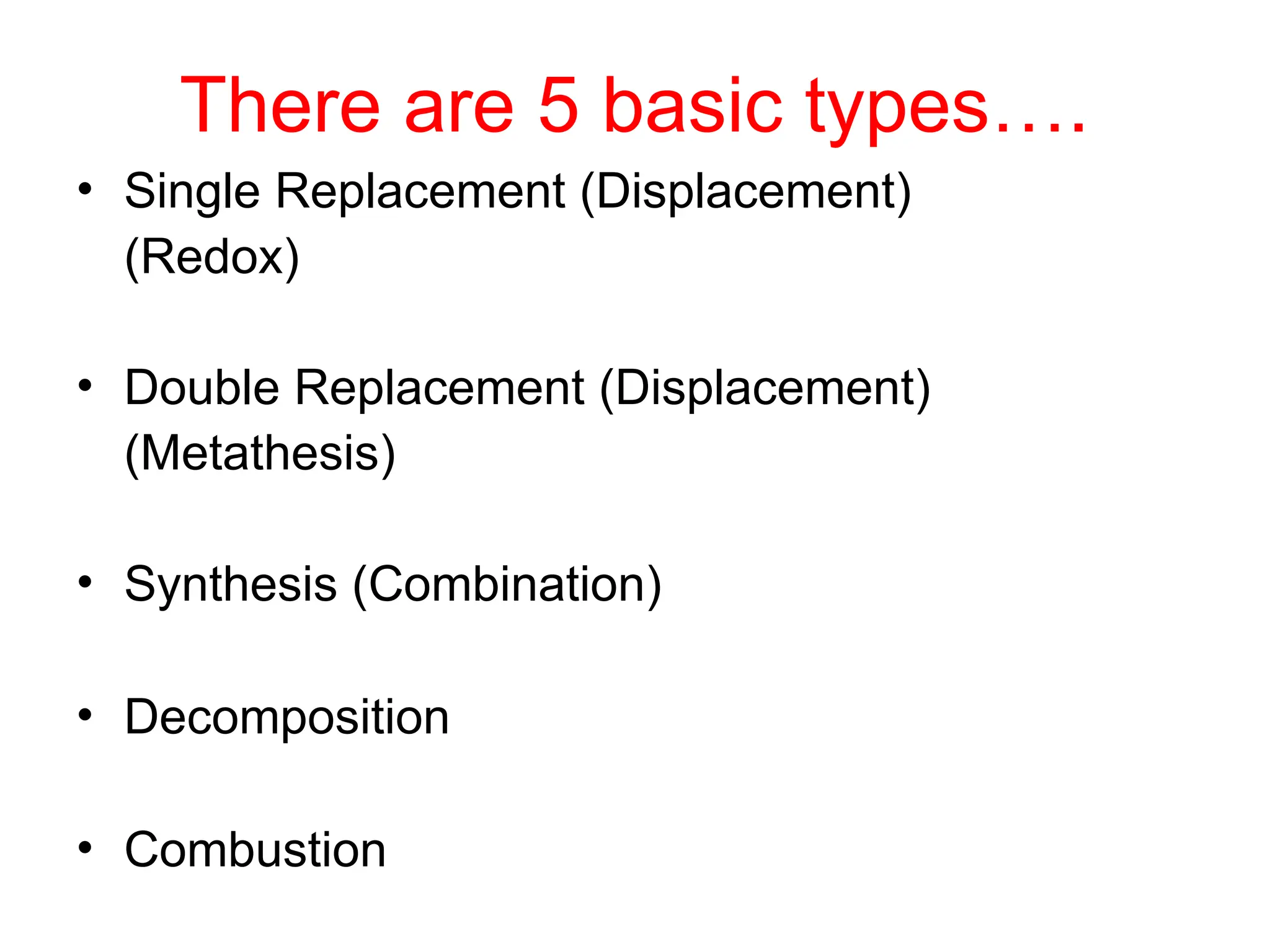
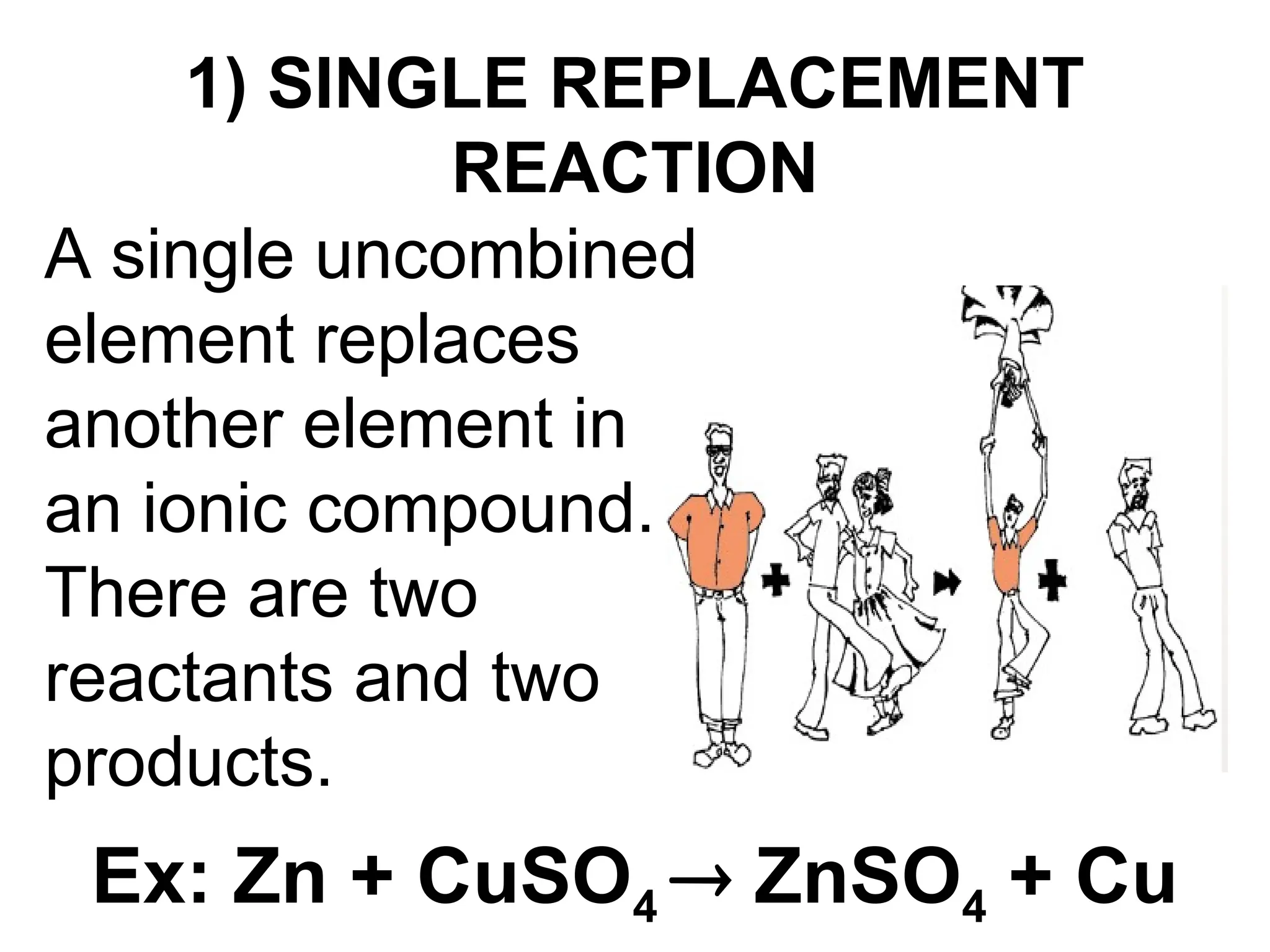
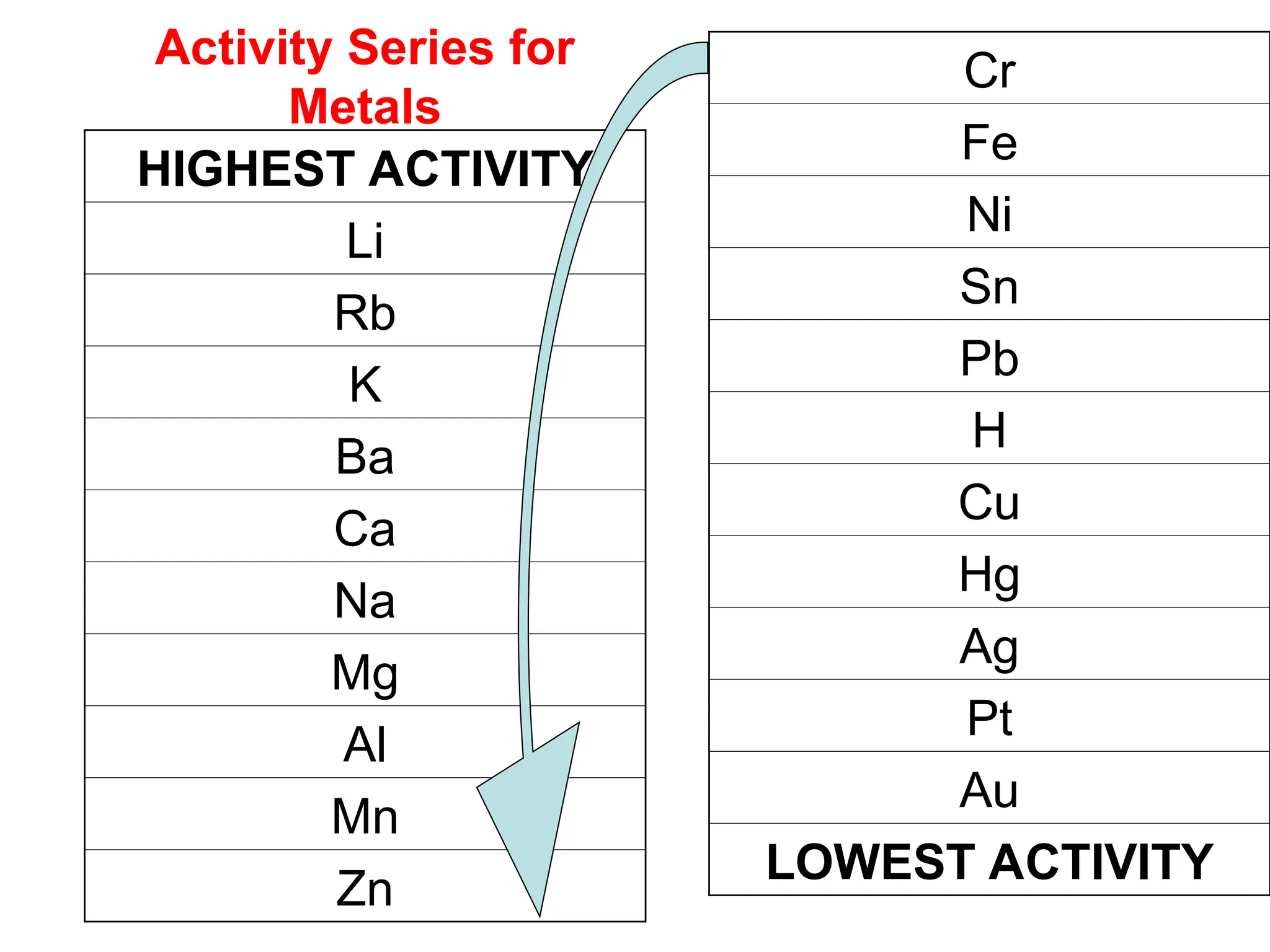
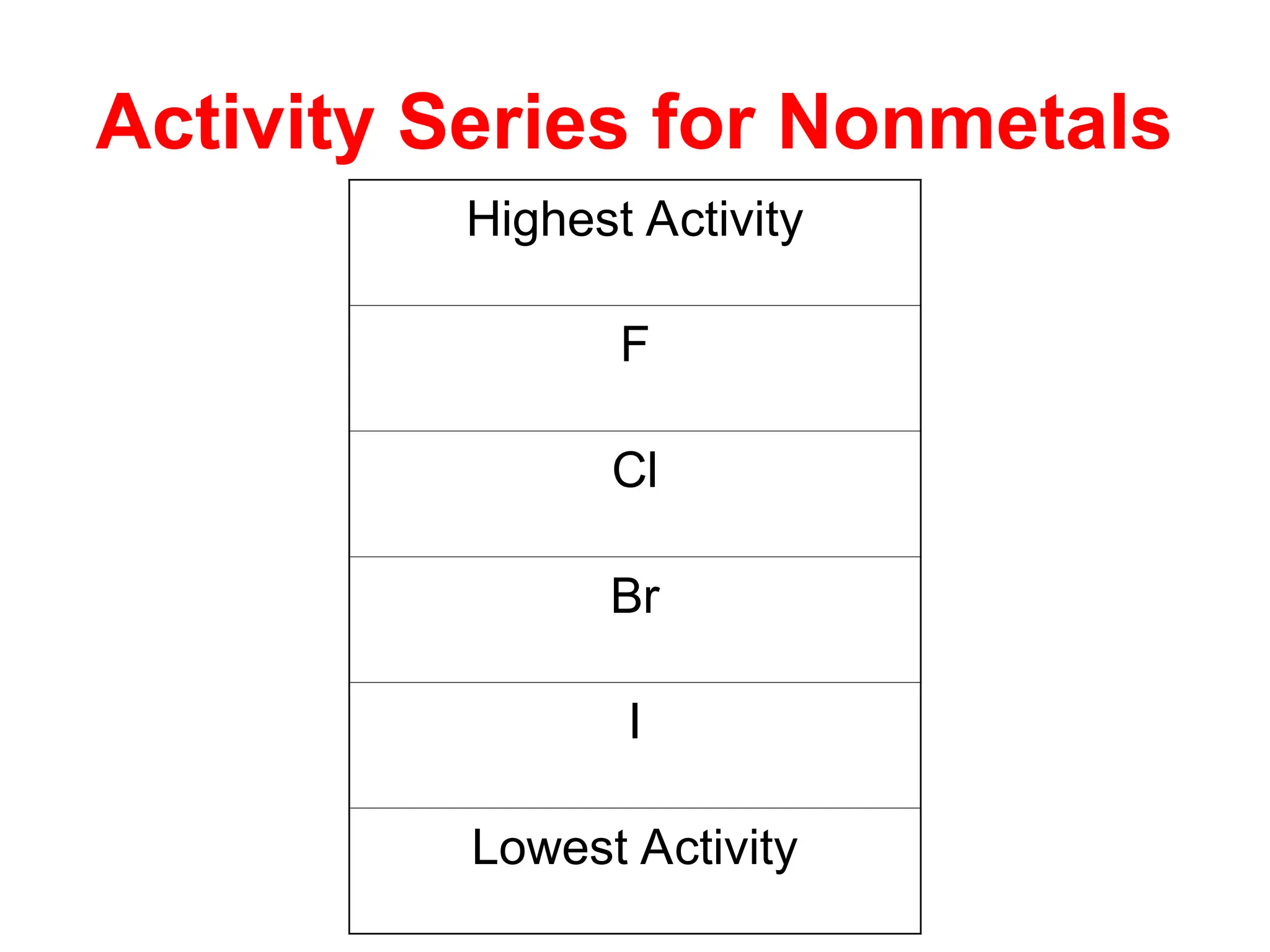
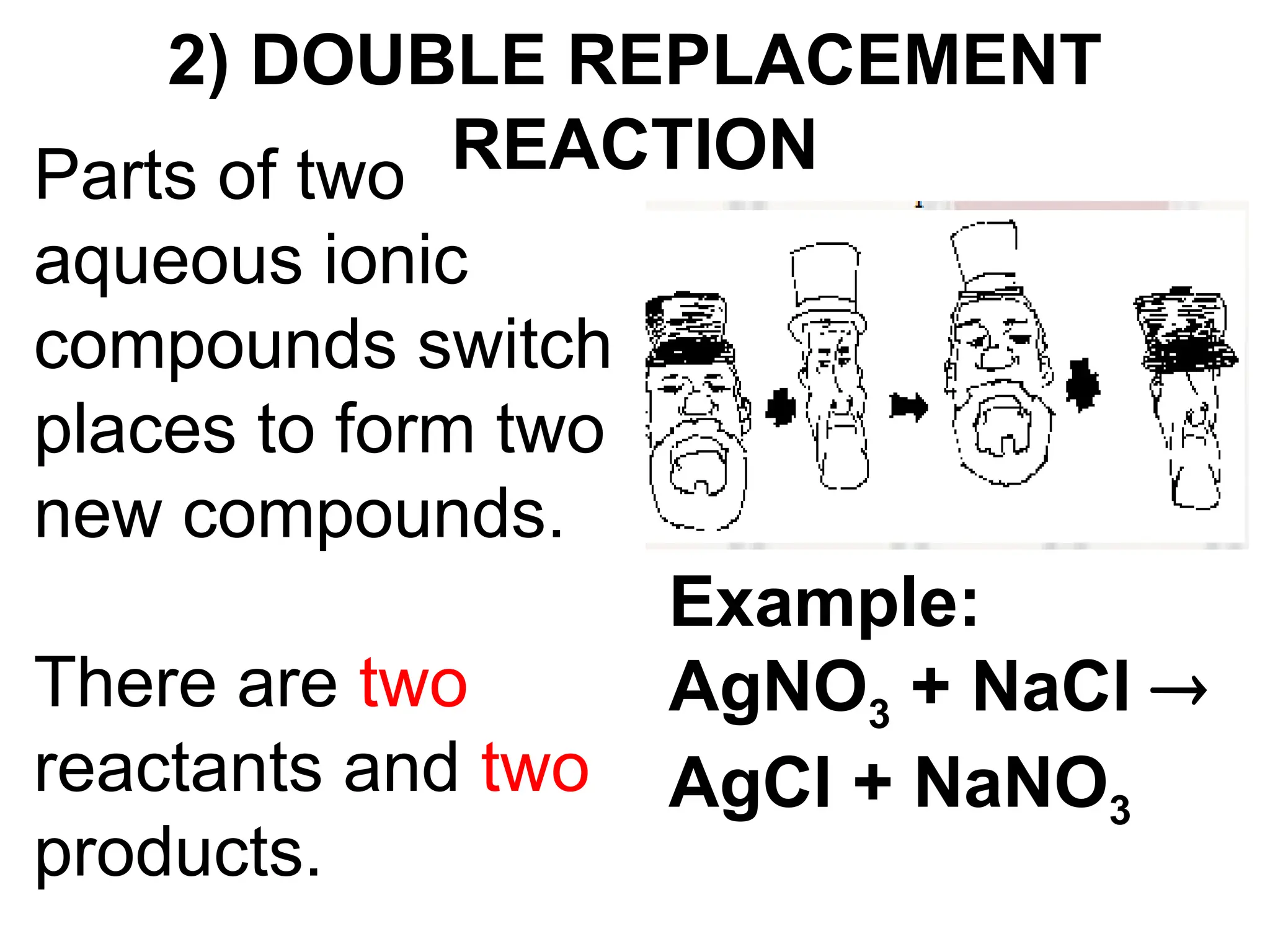
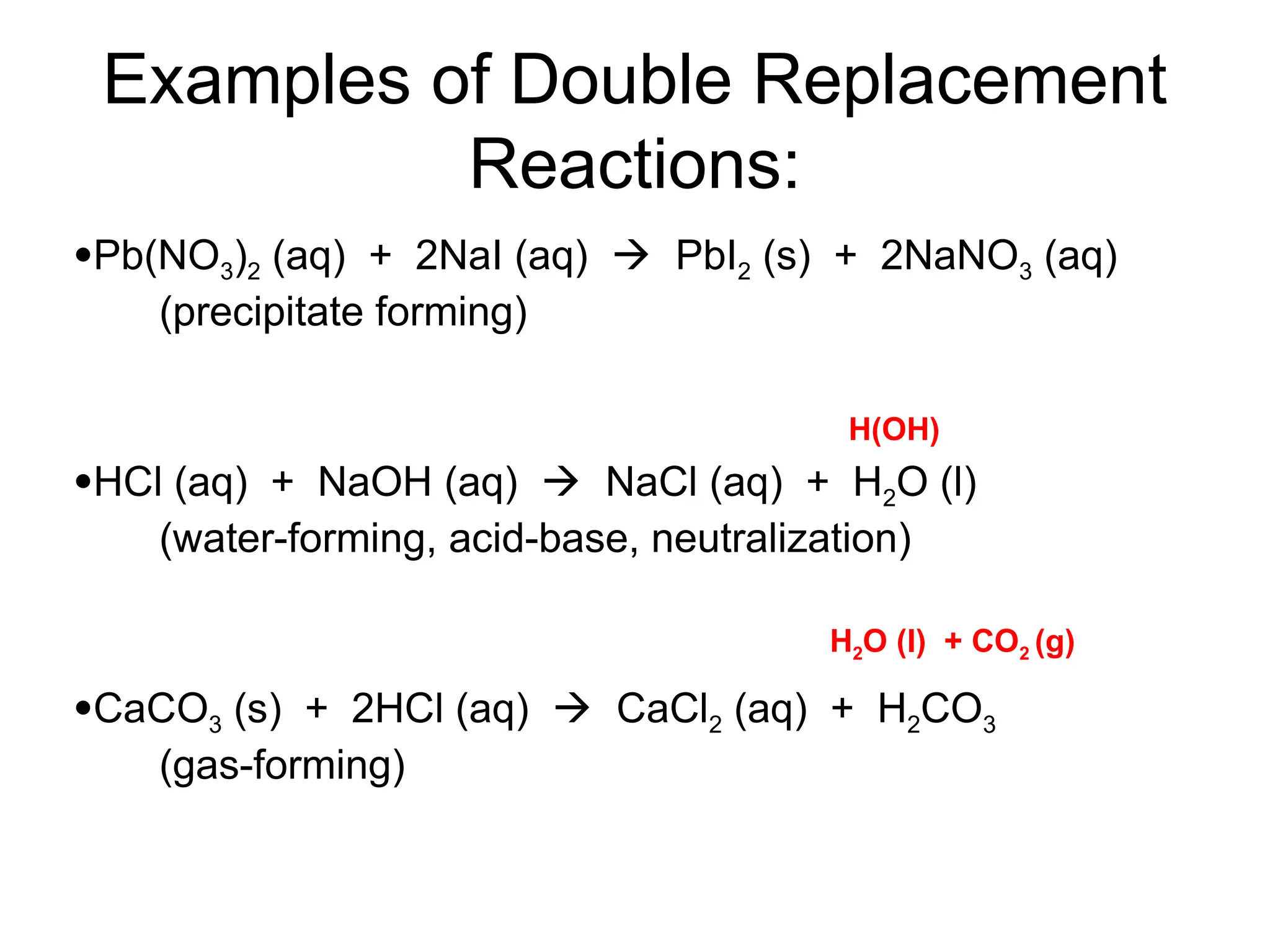
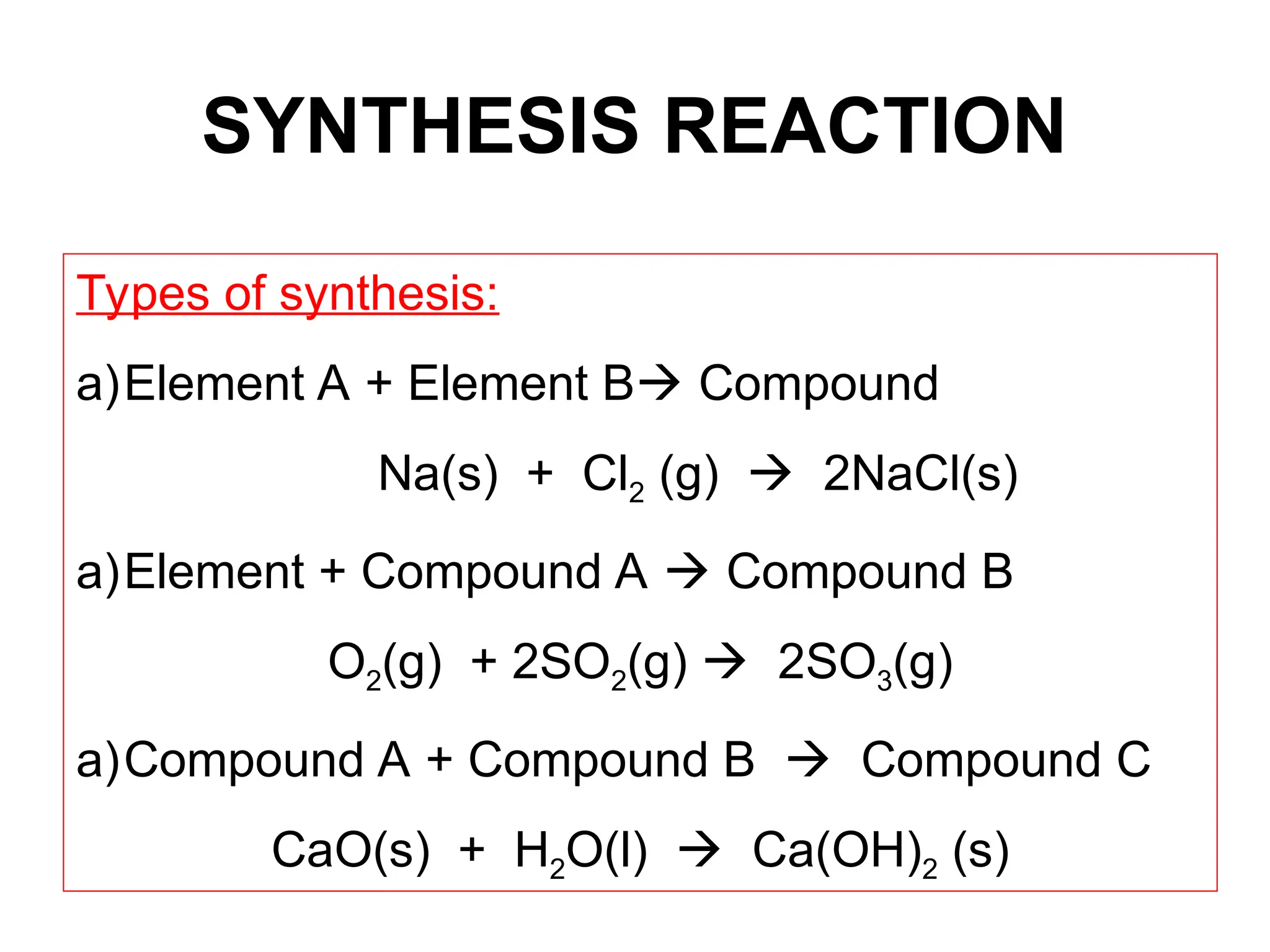
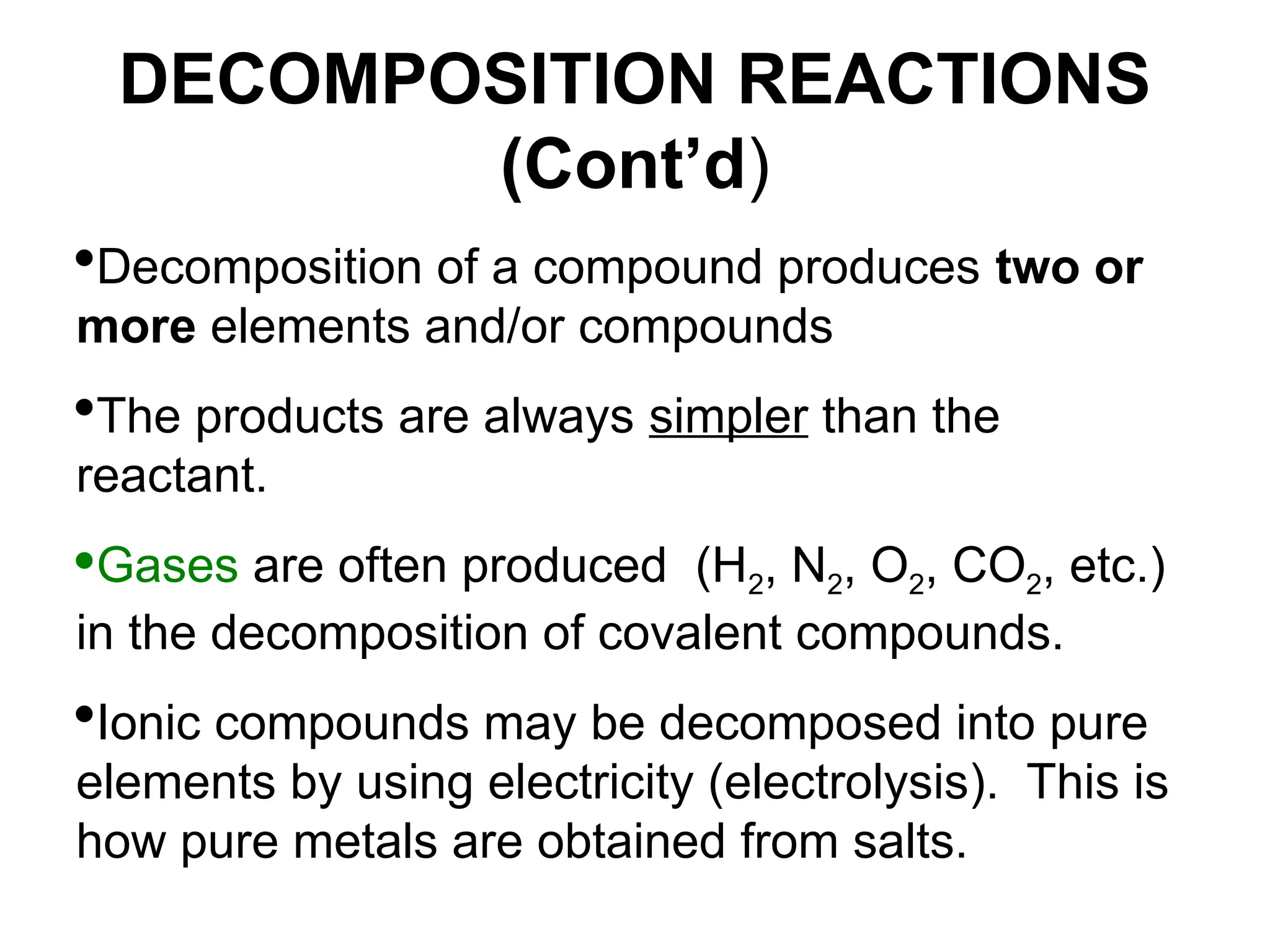
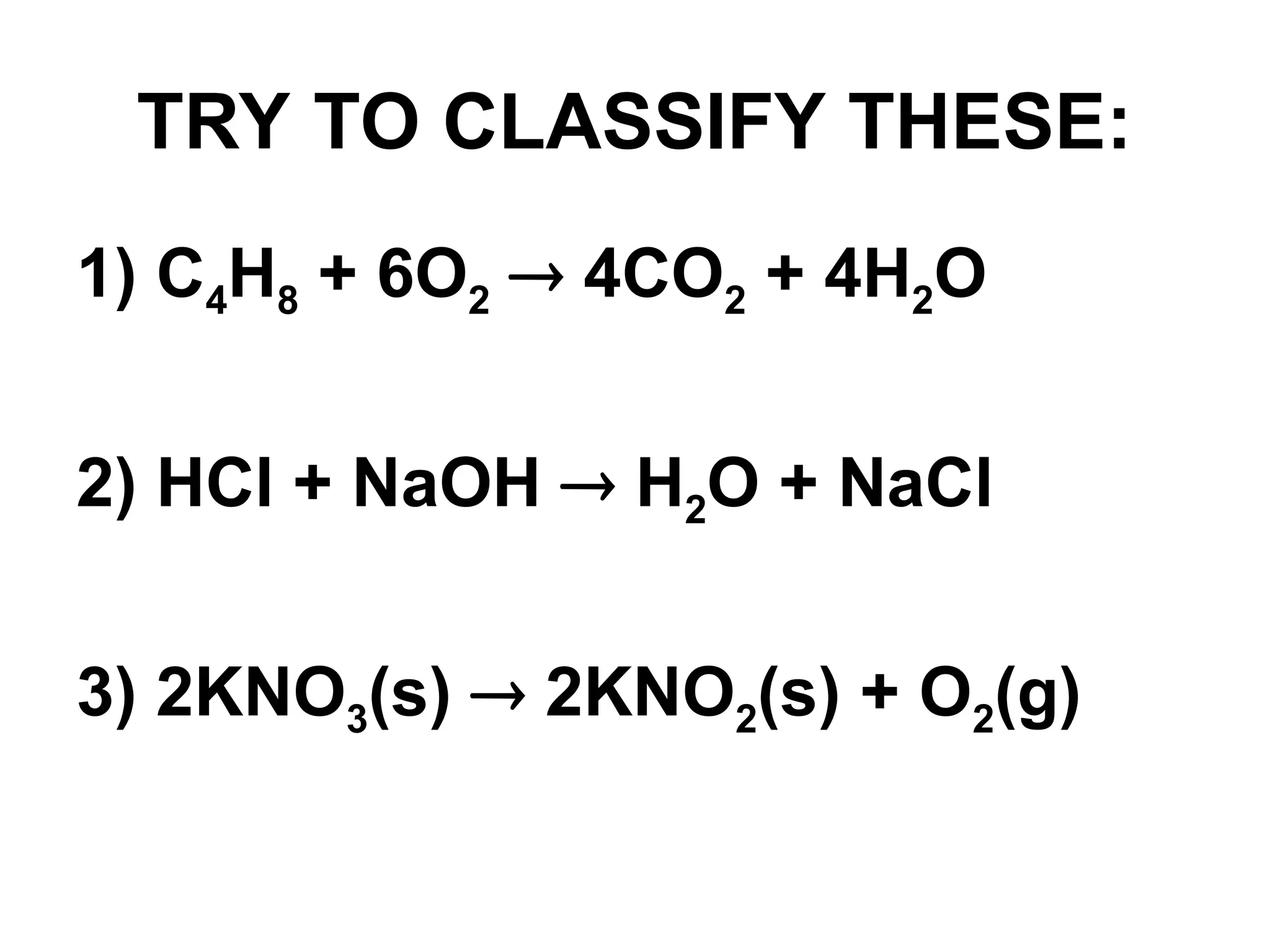
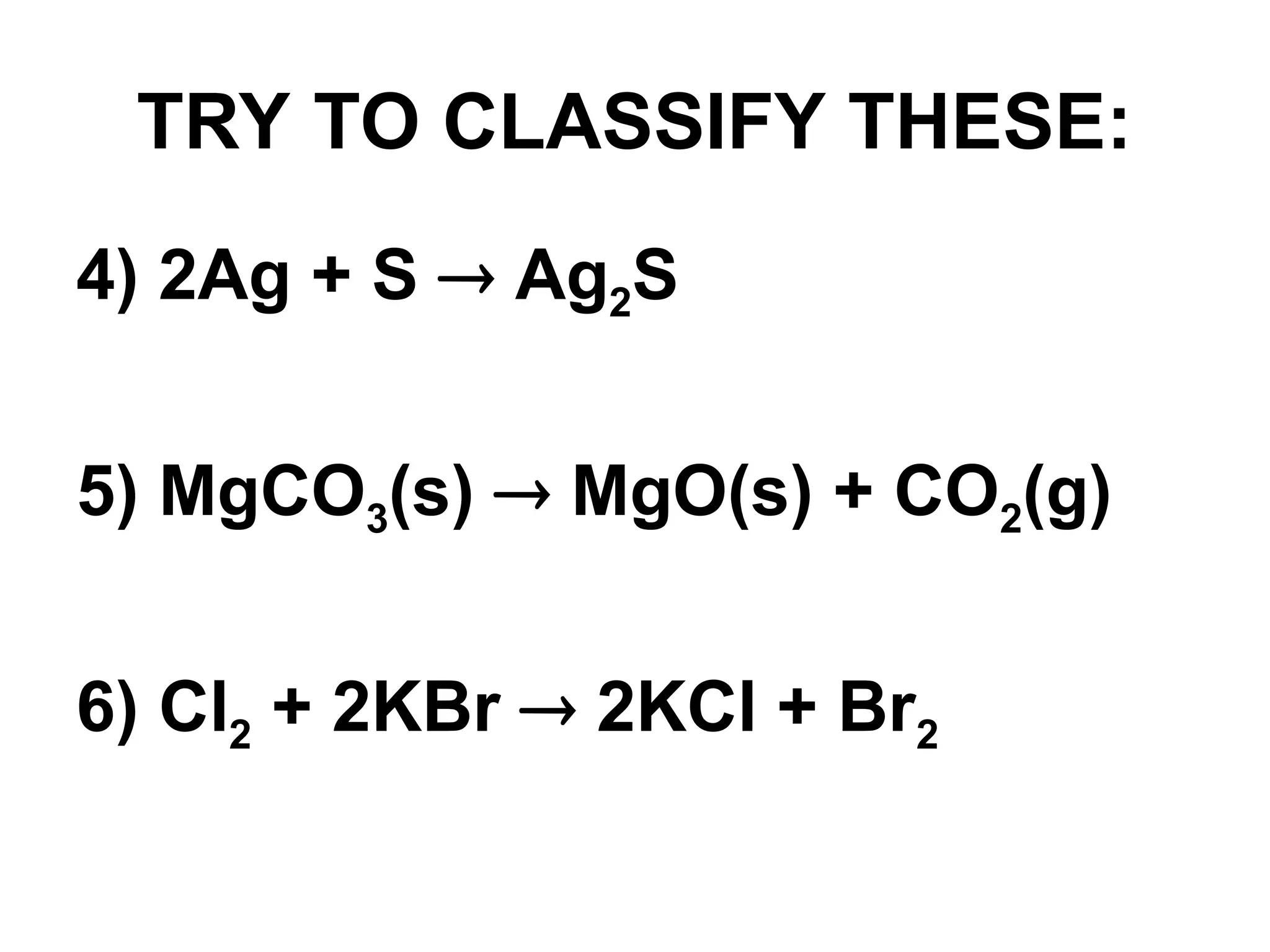
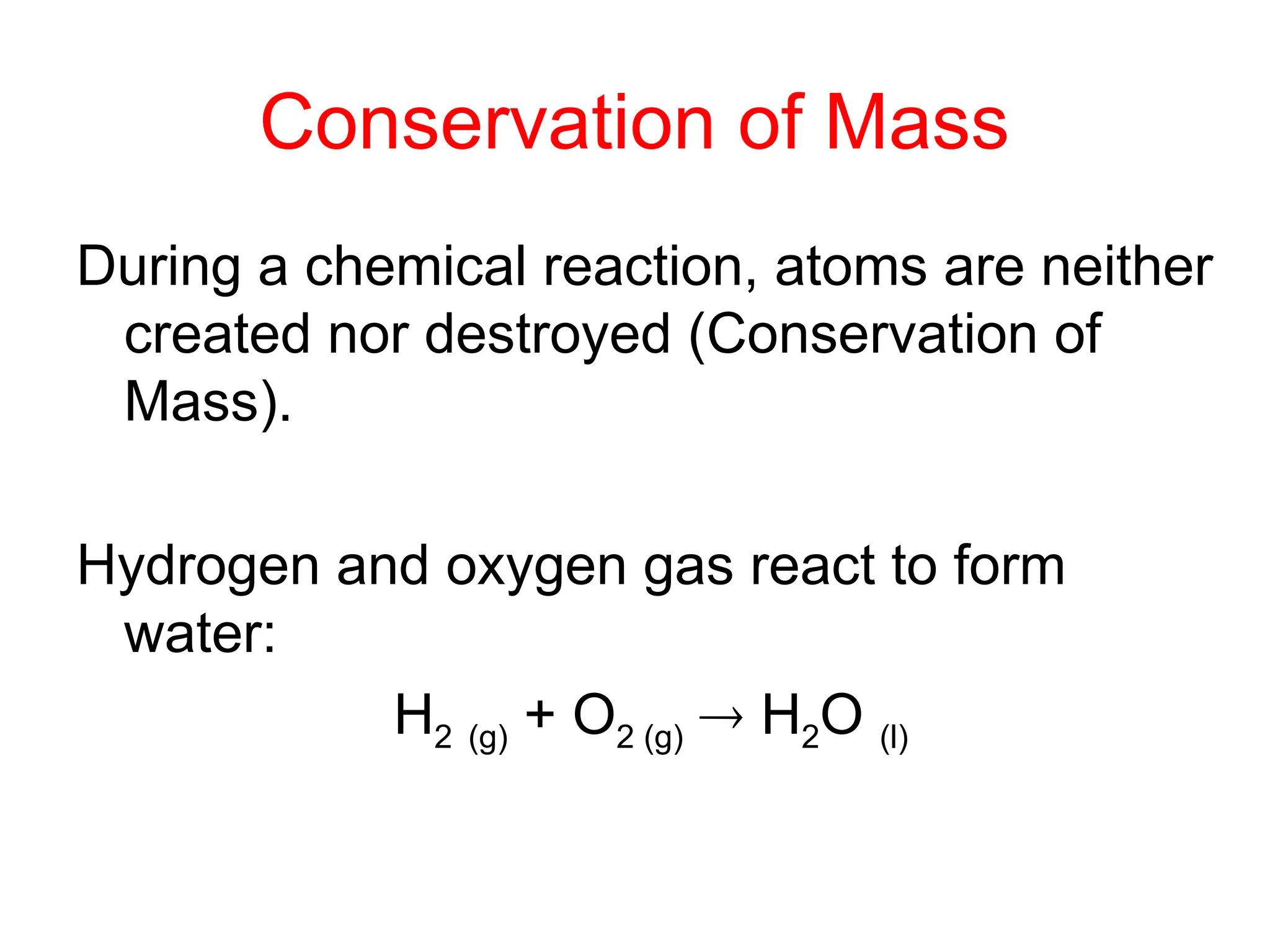
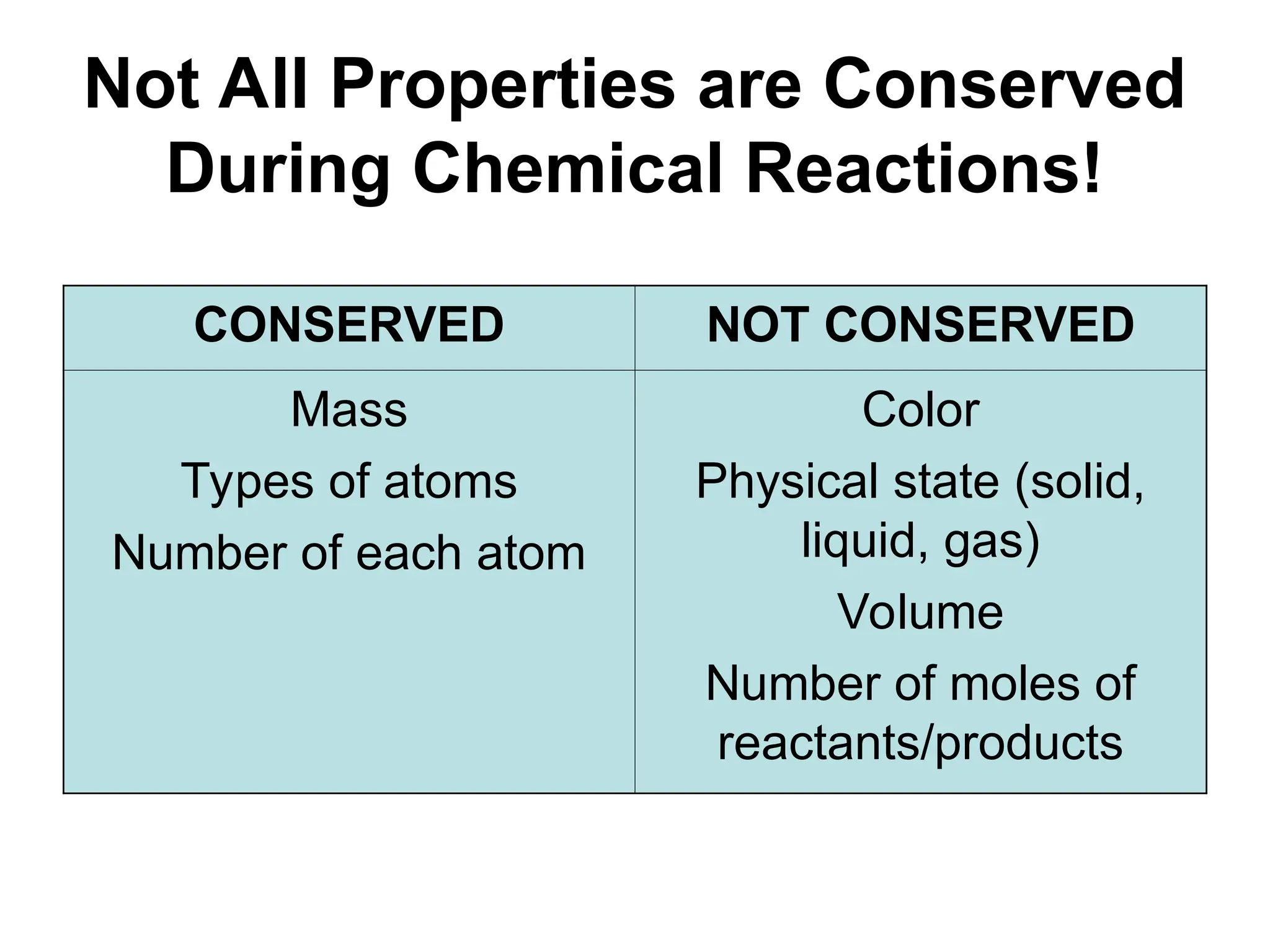
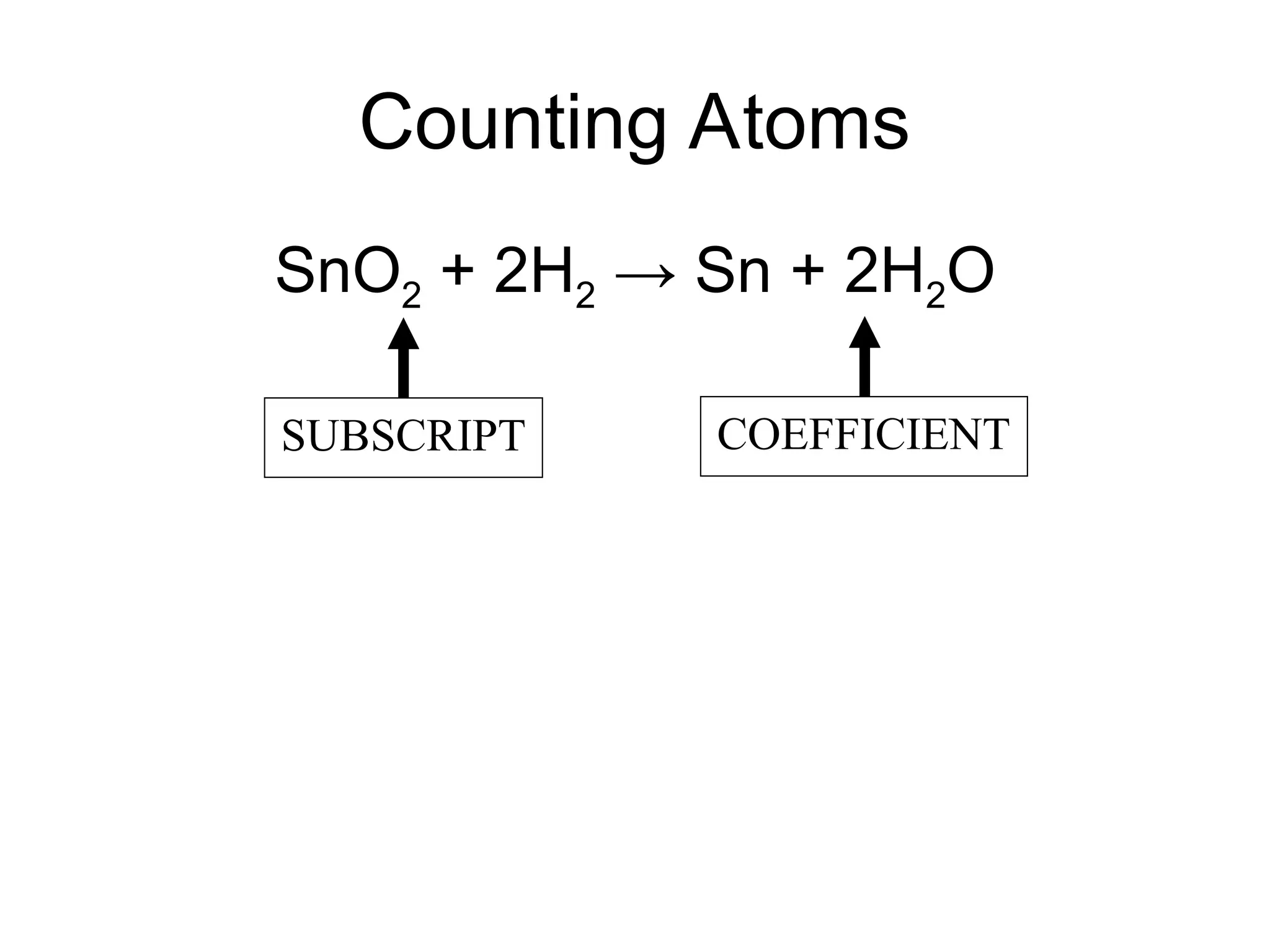
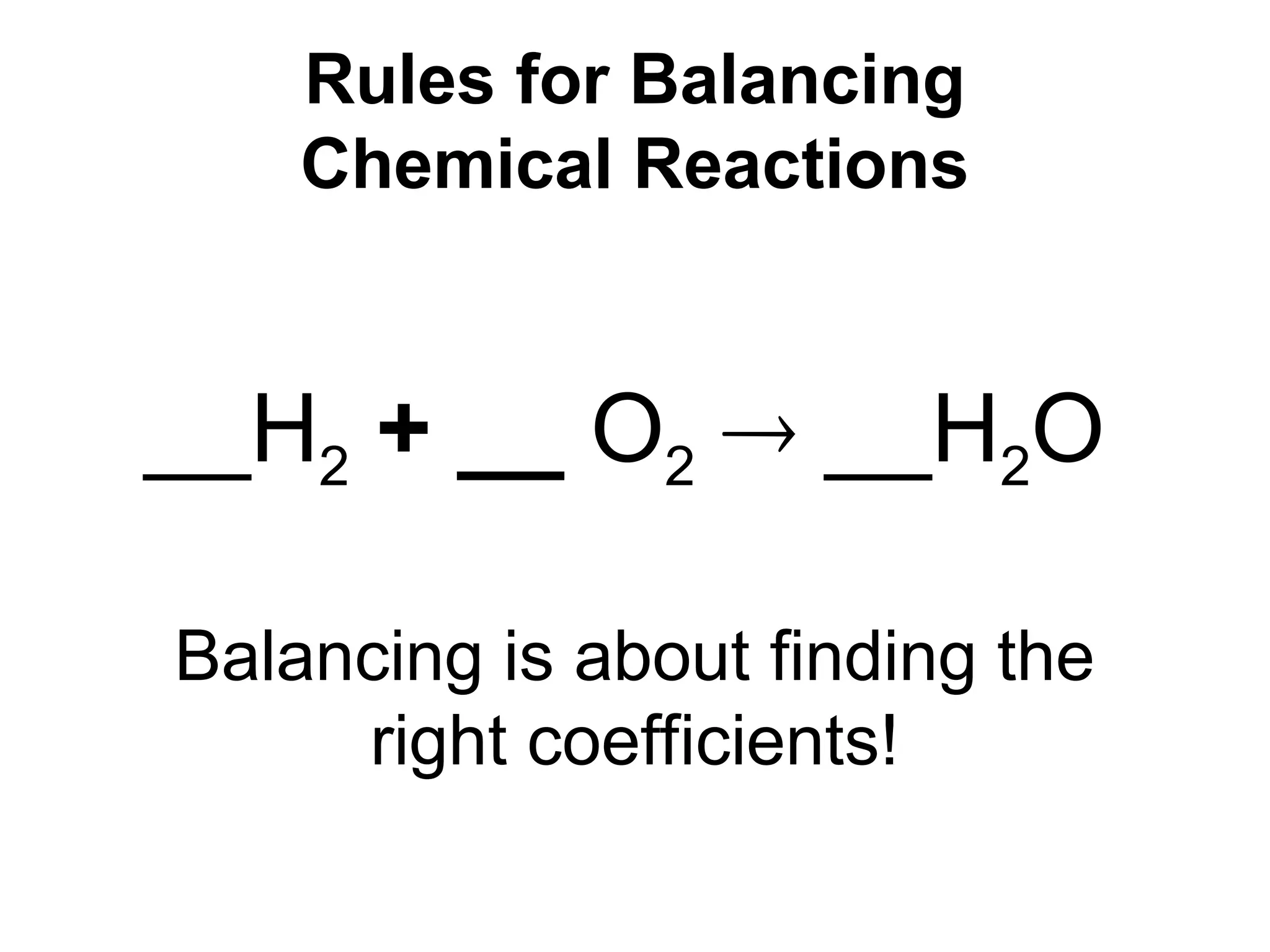
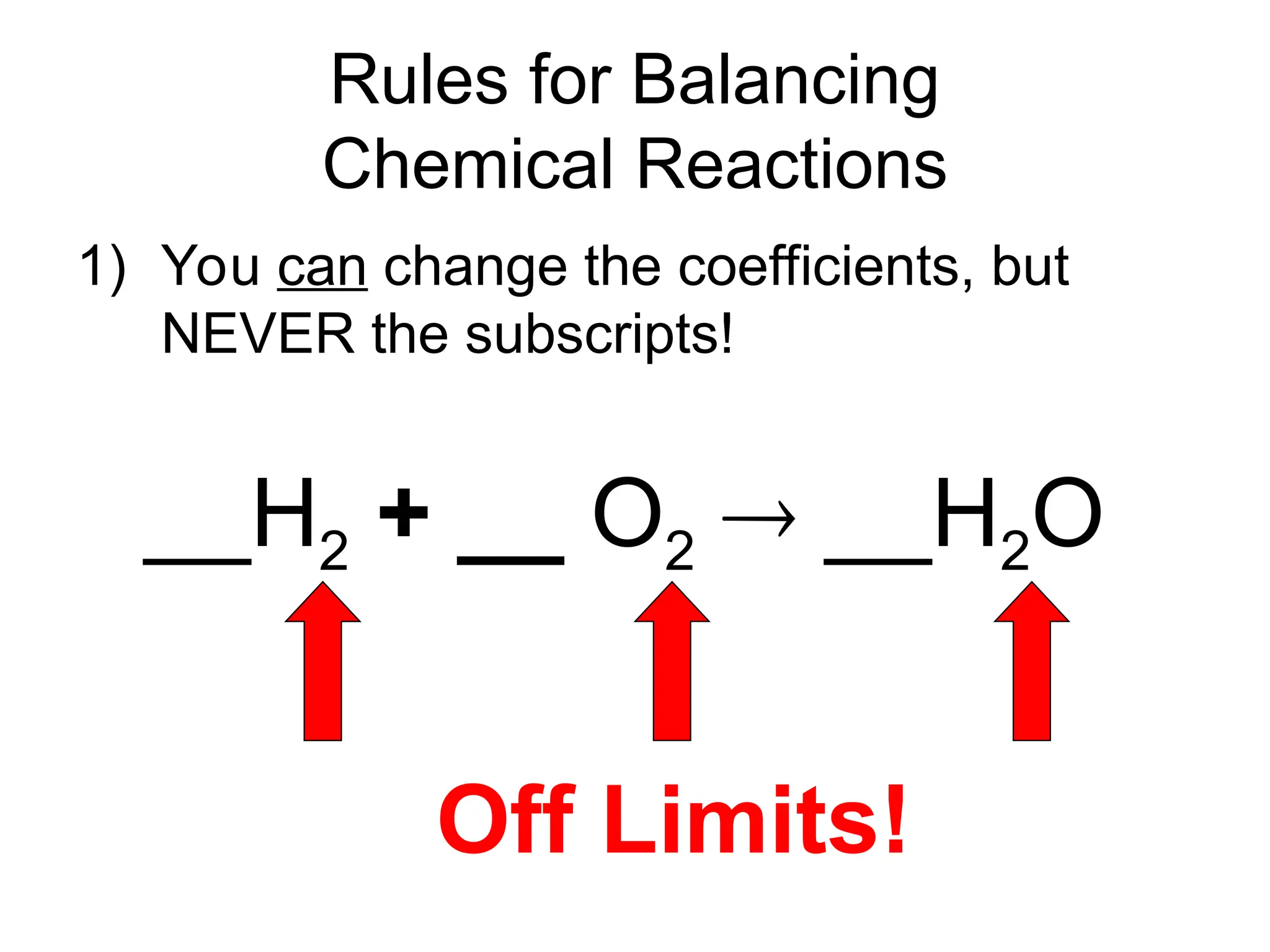
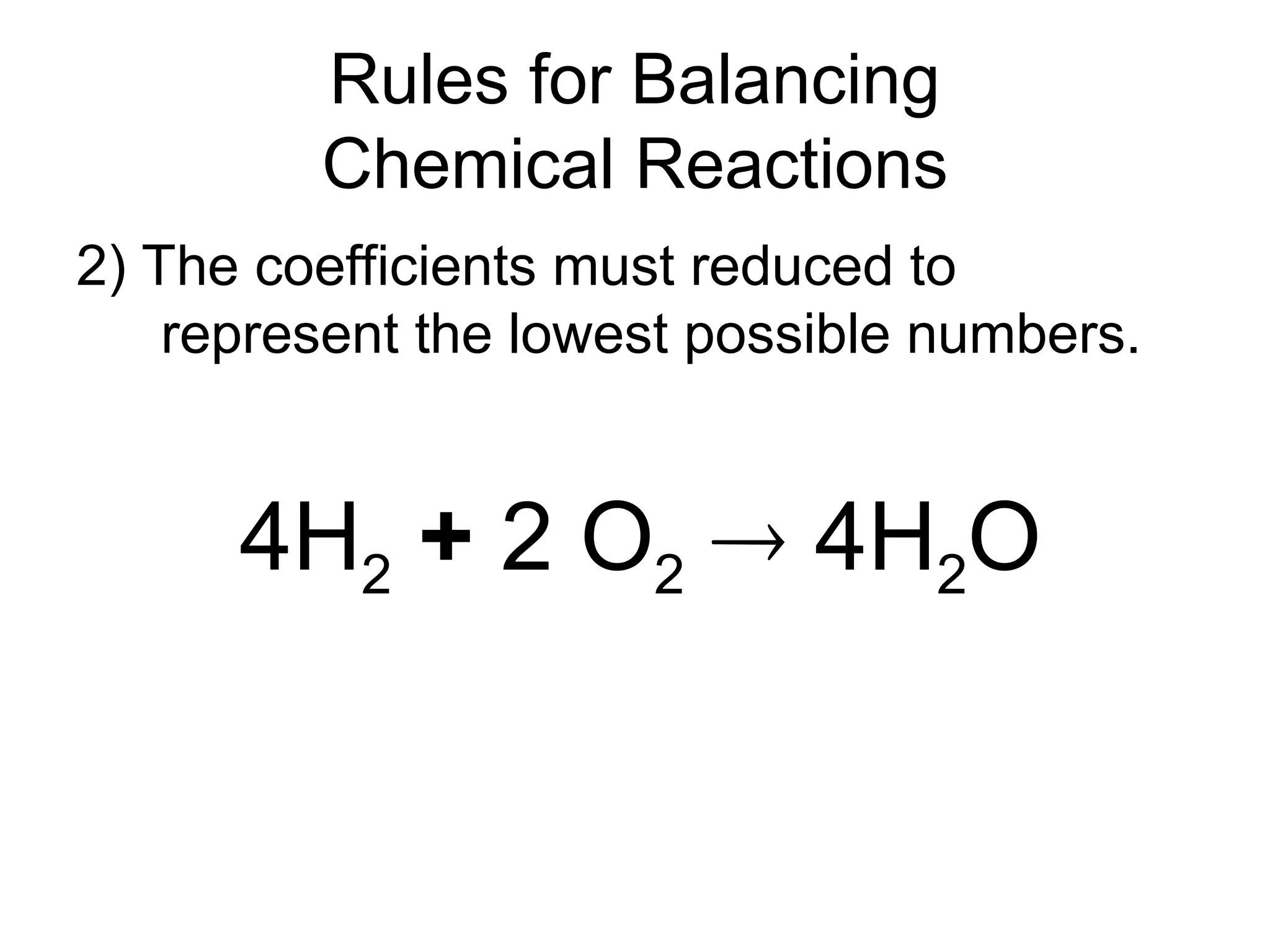
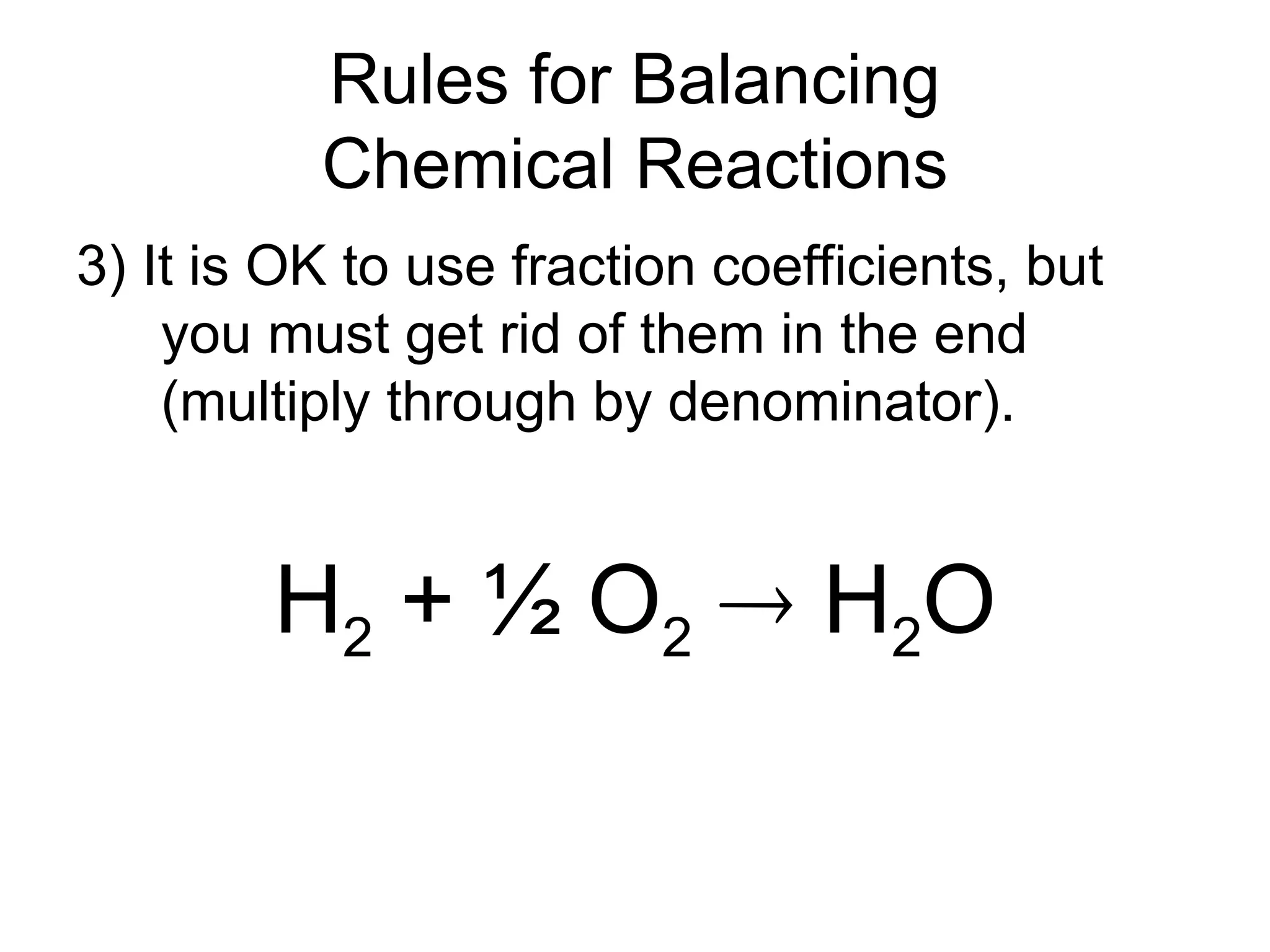
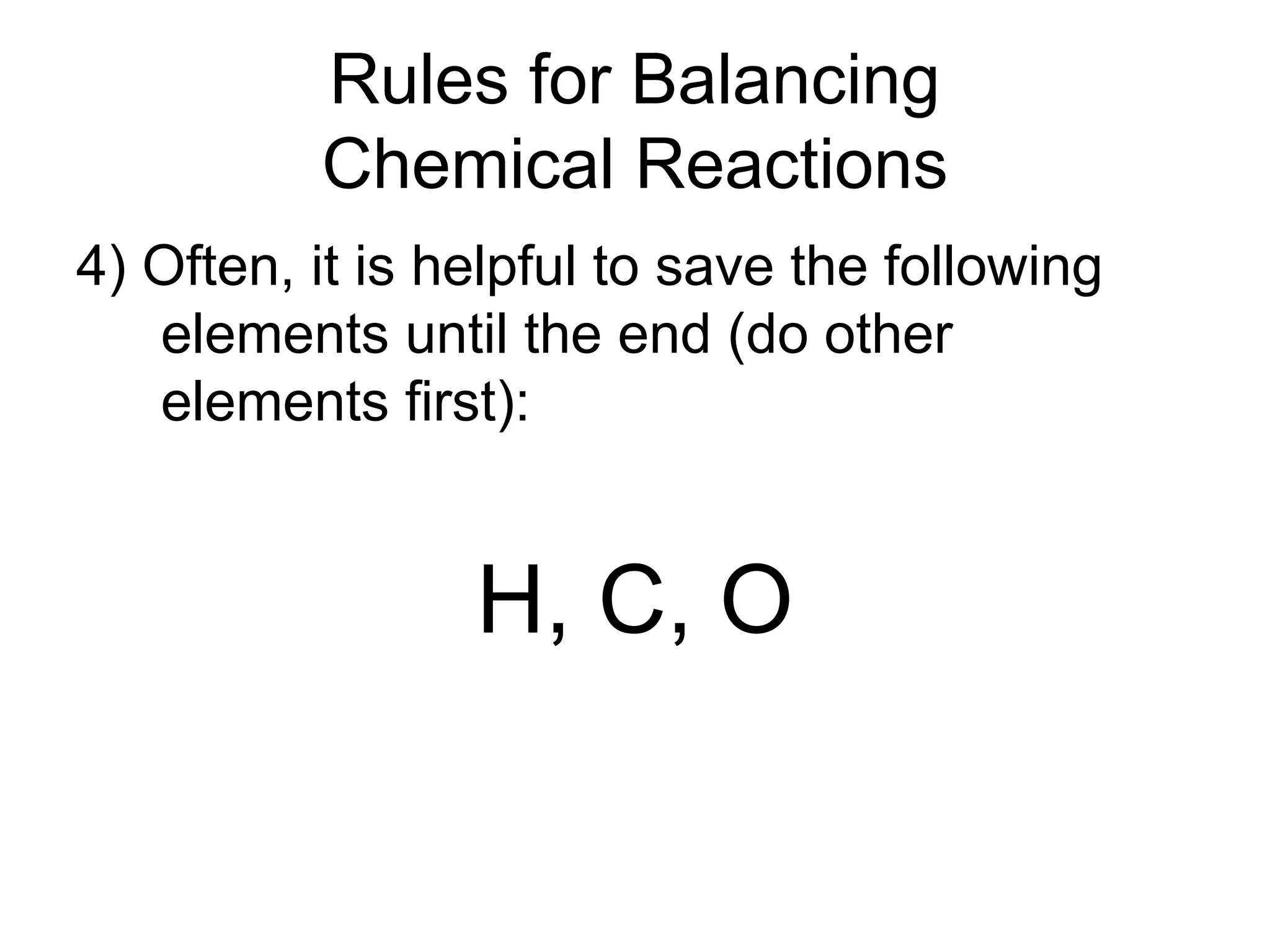
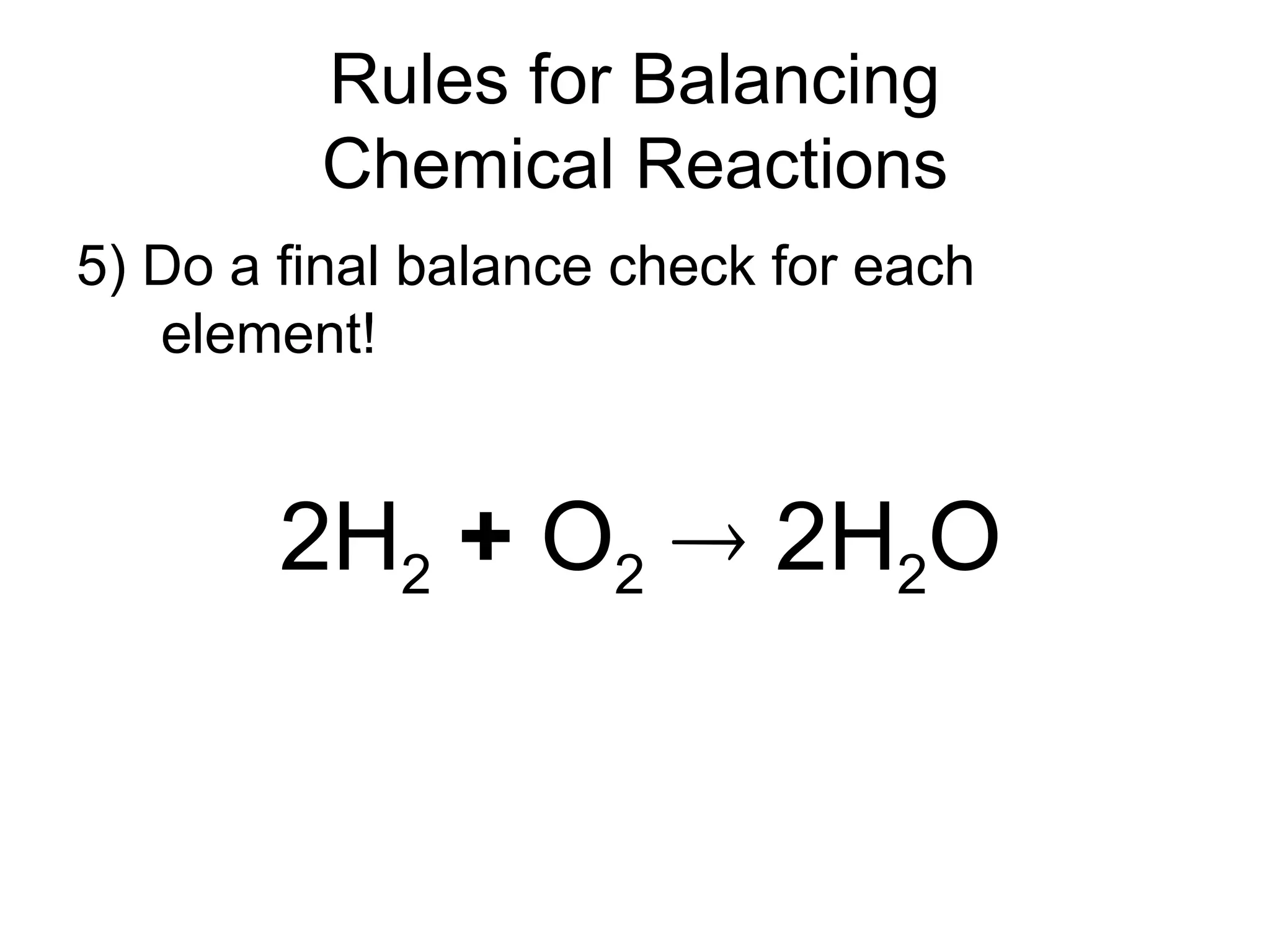
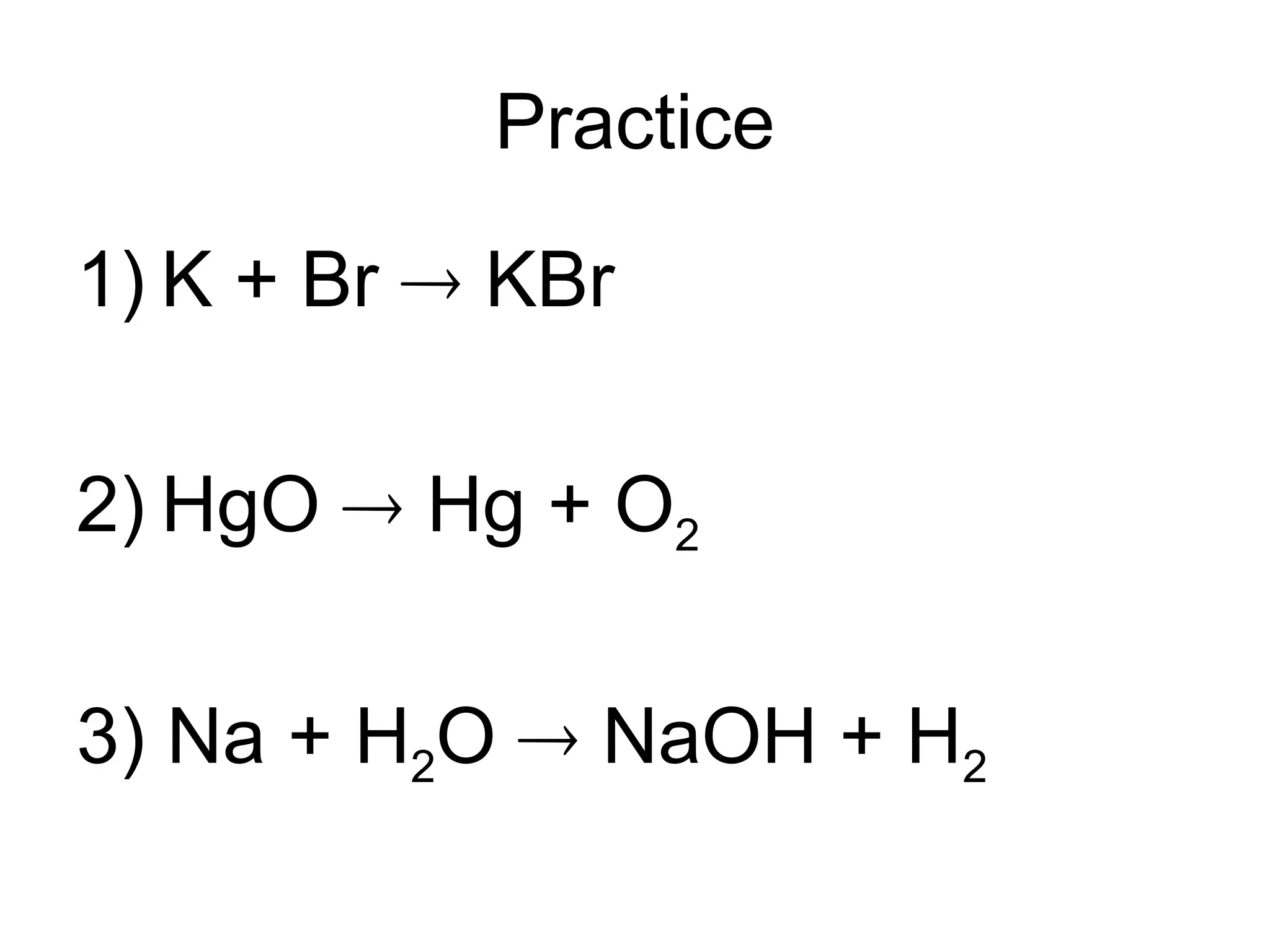
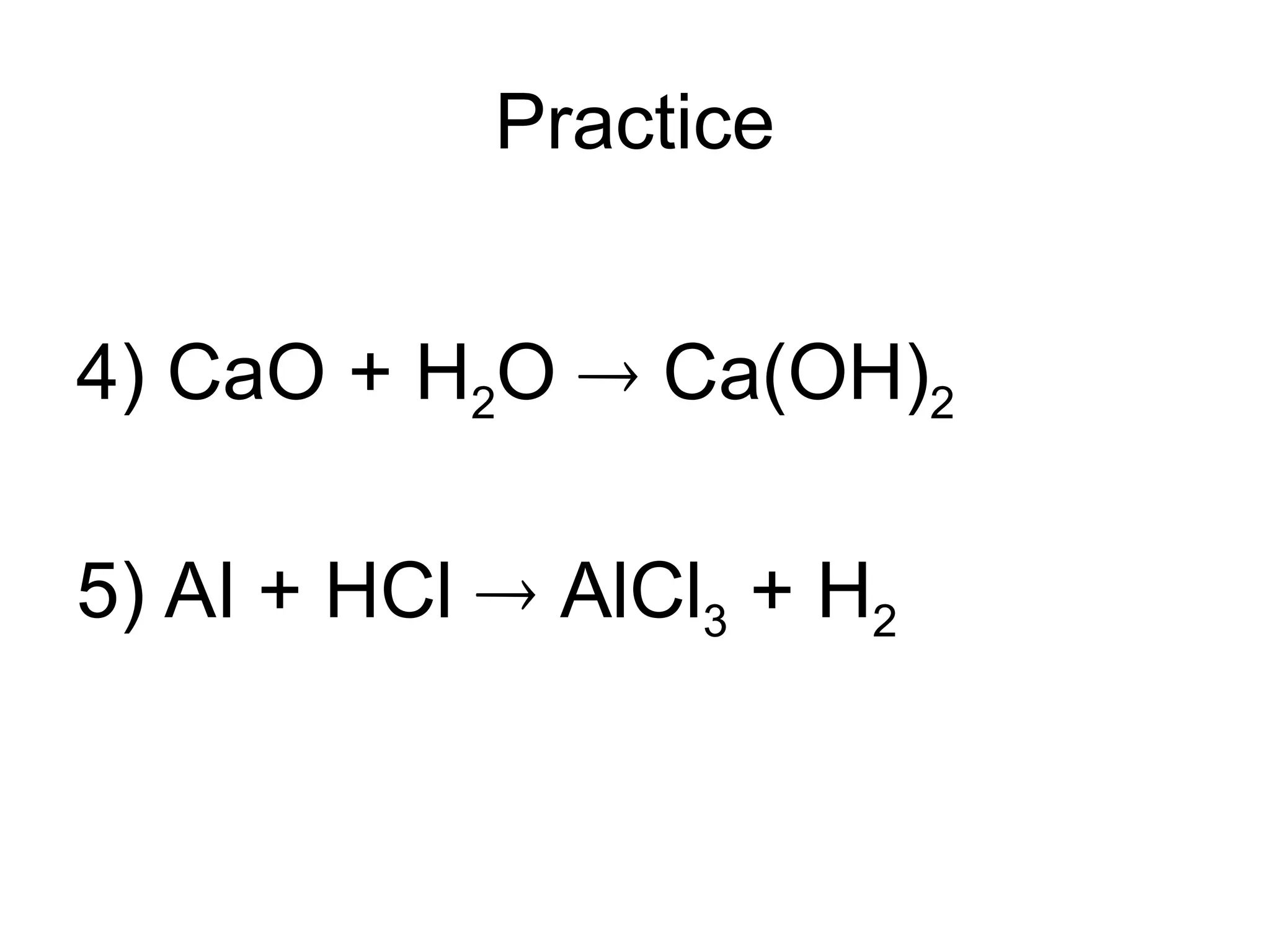
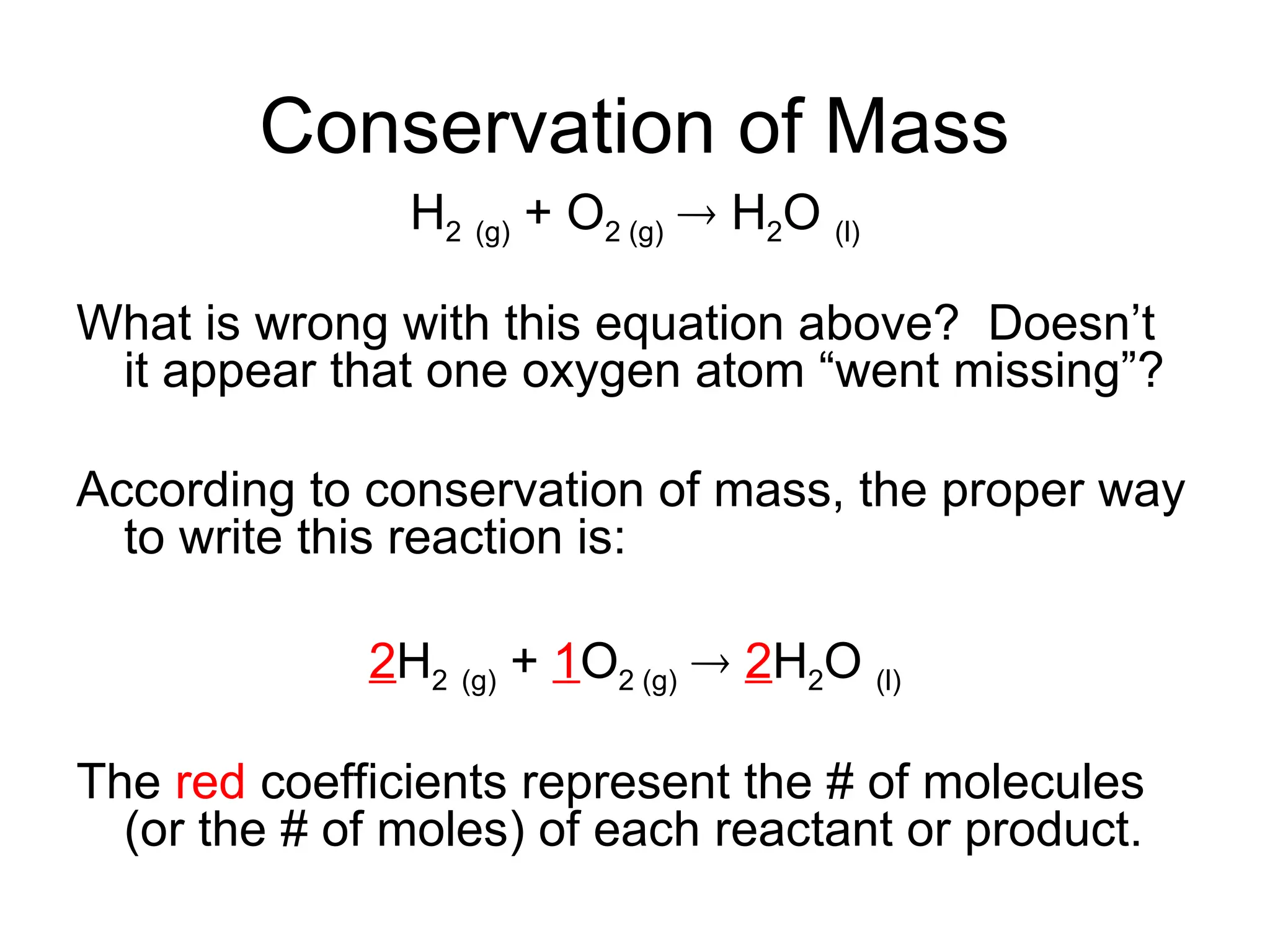This document provides an overview of chemical reactions, defining key concepts such as reactants, products, and various types of reactions including single replacement, double replacement, synthesis, decomposition, and combustion. It explains how to write word equations and skeleton equations, and emphasizes the conservation of mass in reactions, along with guidelines for balancing them. The document also discusses evidence for chemical reactions and the activity series that determines whether certain reactions occur.