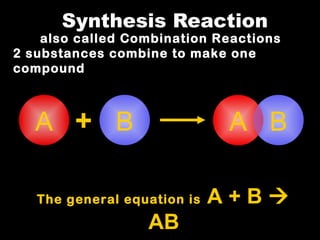
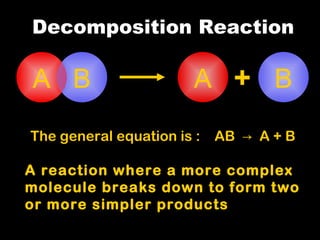
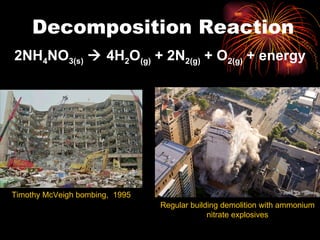
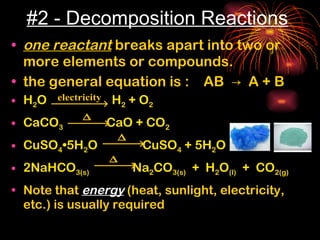
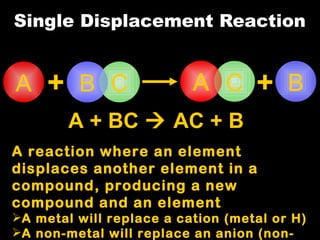
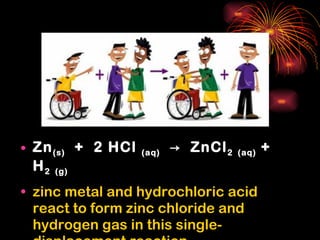
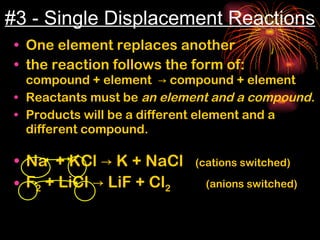
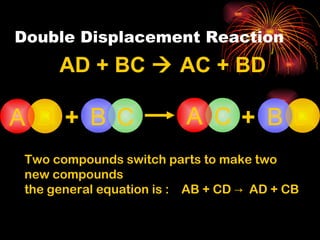
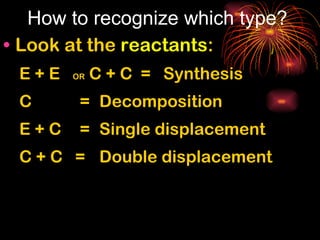
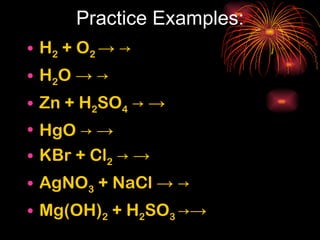
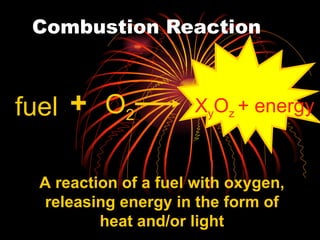
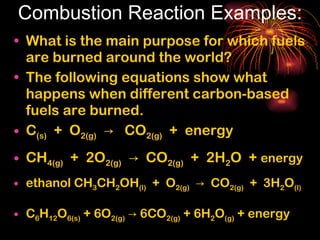
Chemical reactions involve reactants transforming into products. There are several types of chemical reactions that can be identified by their reactants and products. These include synthesis reactions where two reactants combine to form one product, decomposition reactions where one reactant breaks down into multiple products, and displacement reactions where one element replaces another in a compound. Balancing chemical equations ensures the same number and type of atoms are on both sides of the reaction.










































![Predicting the Precipitate Insoluble salt = a precipitate [note Figure 11.11, p.342 (AgCl)] General solubility rules are found: Table 11.3, p. 344 in textbook Reference section - page R54 (back of textbook) Lab manual Table A.3, page 332 Your periodic table handout](https://image.slidesharecdn.com/balancingandreactiontypes-110329100127-phpapp01/85/Balancing-and-reaction-types-56-320.jpg)
