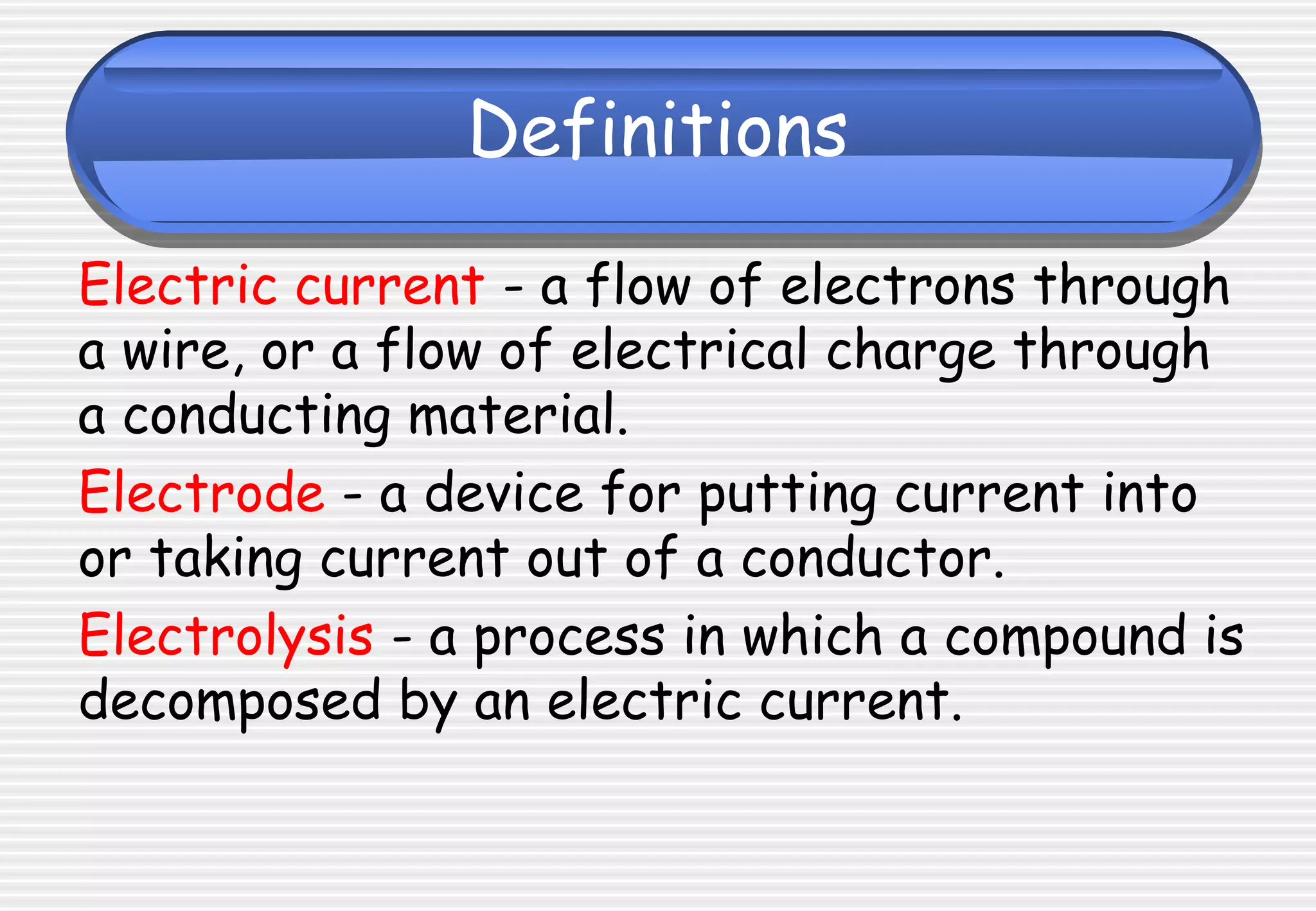
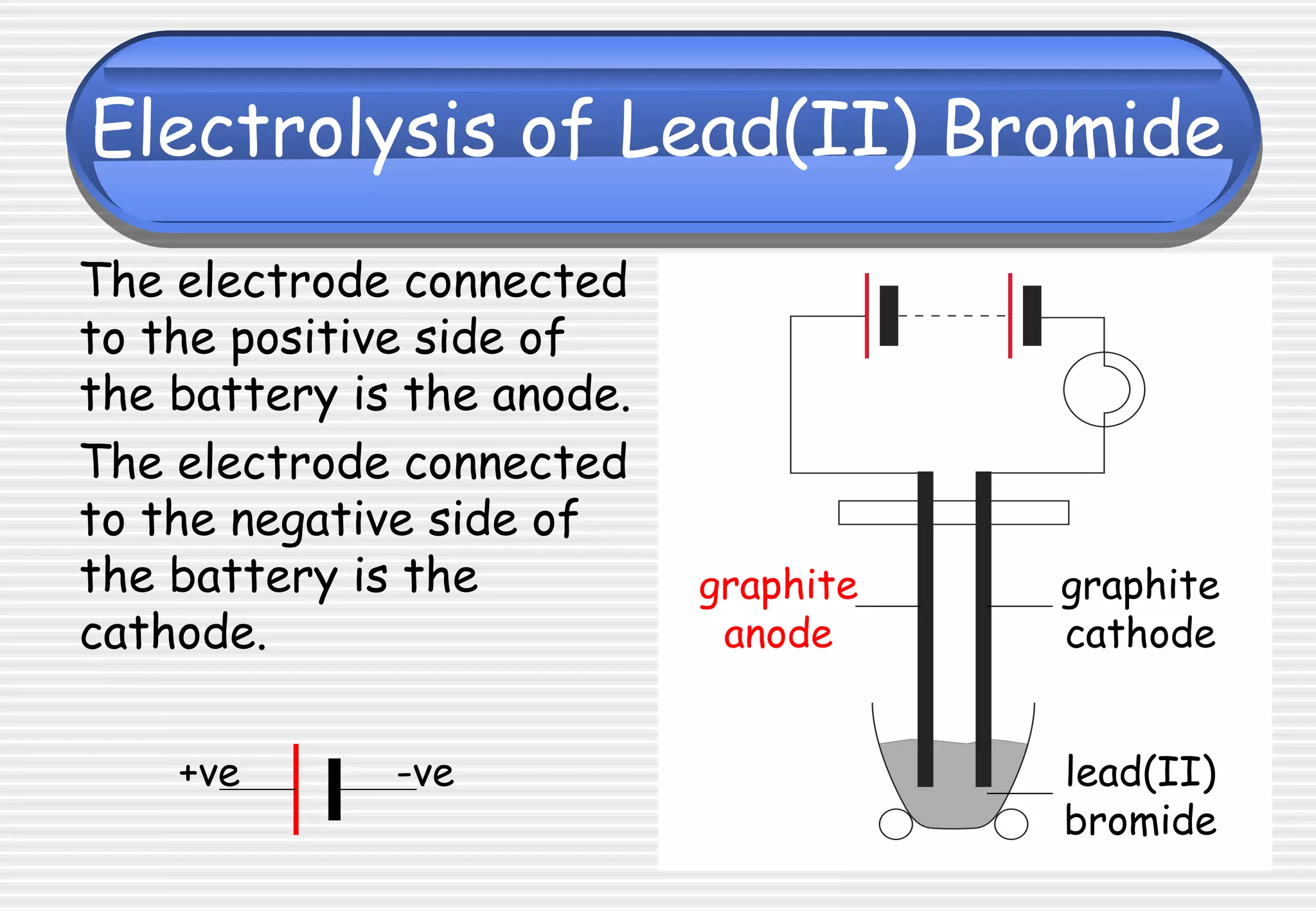
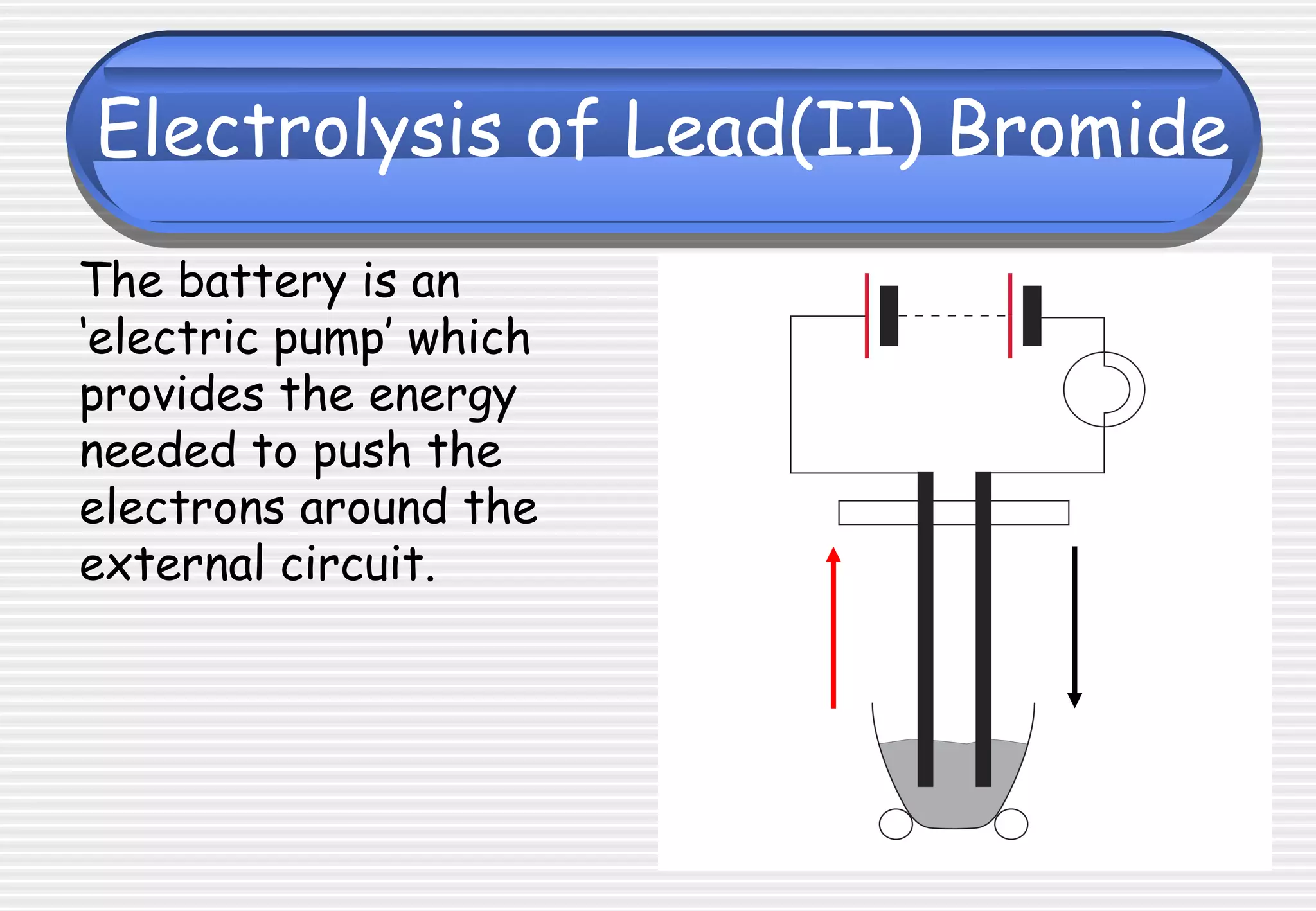
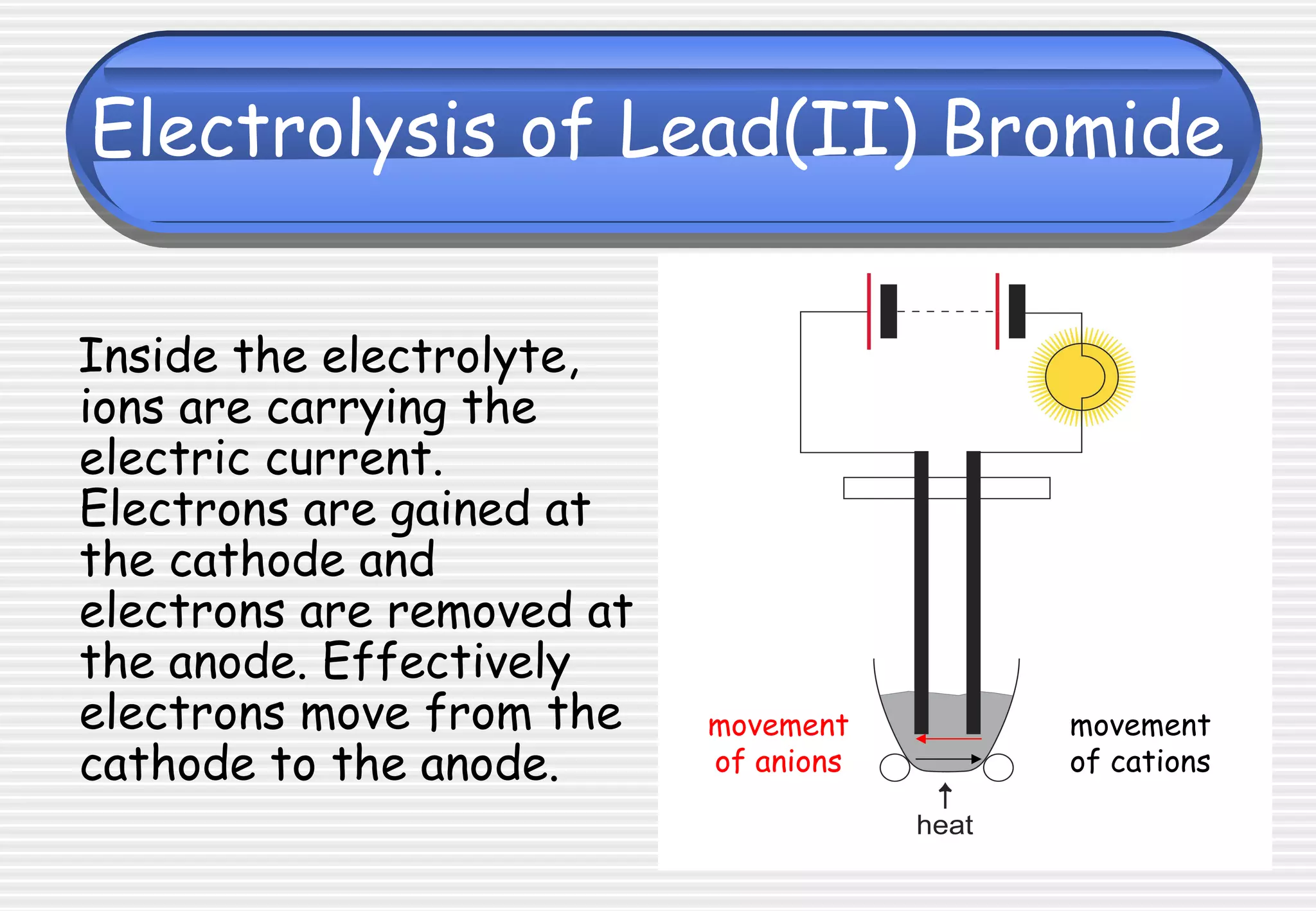
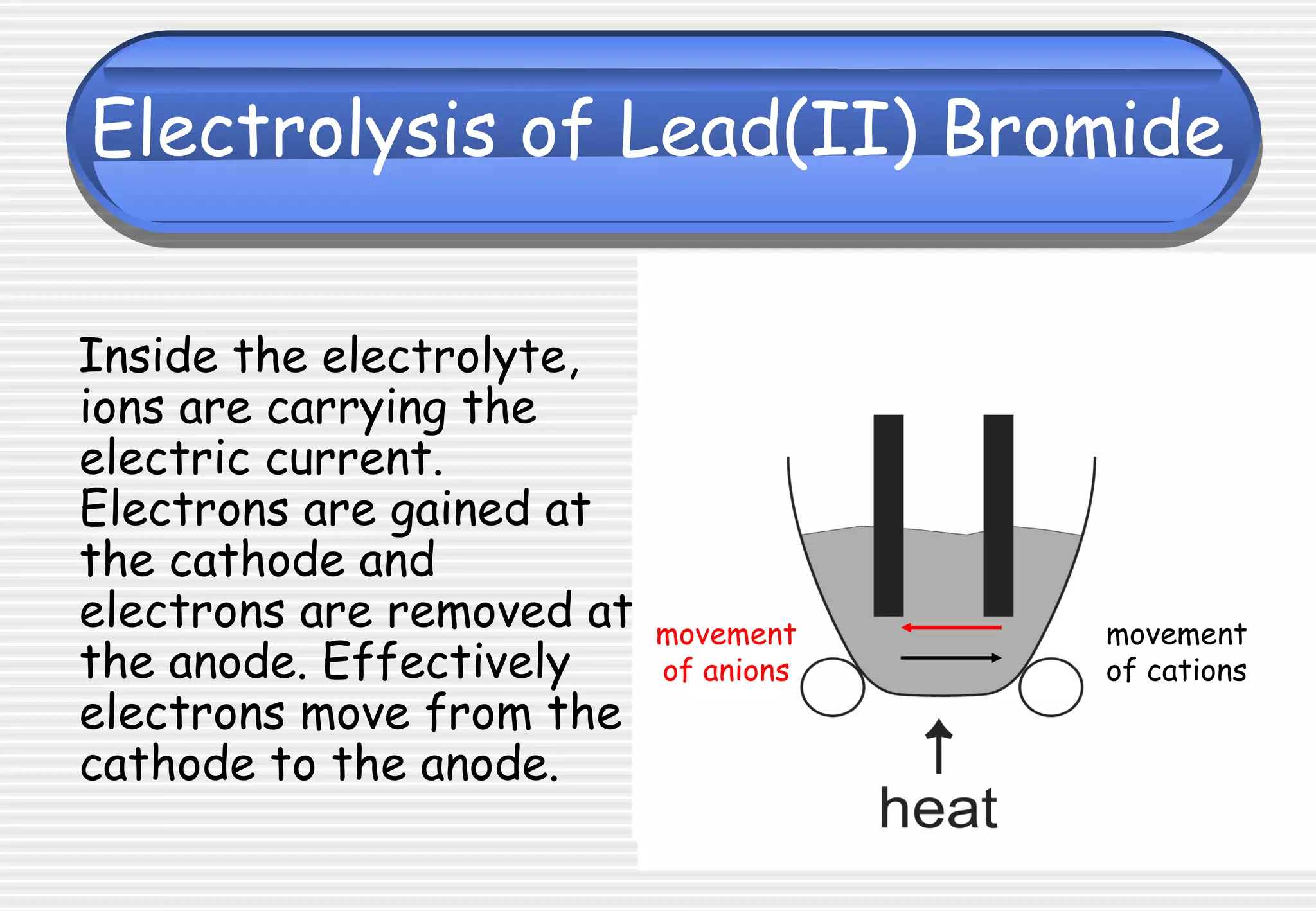
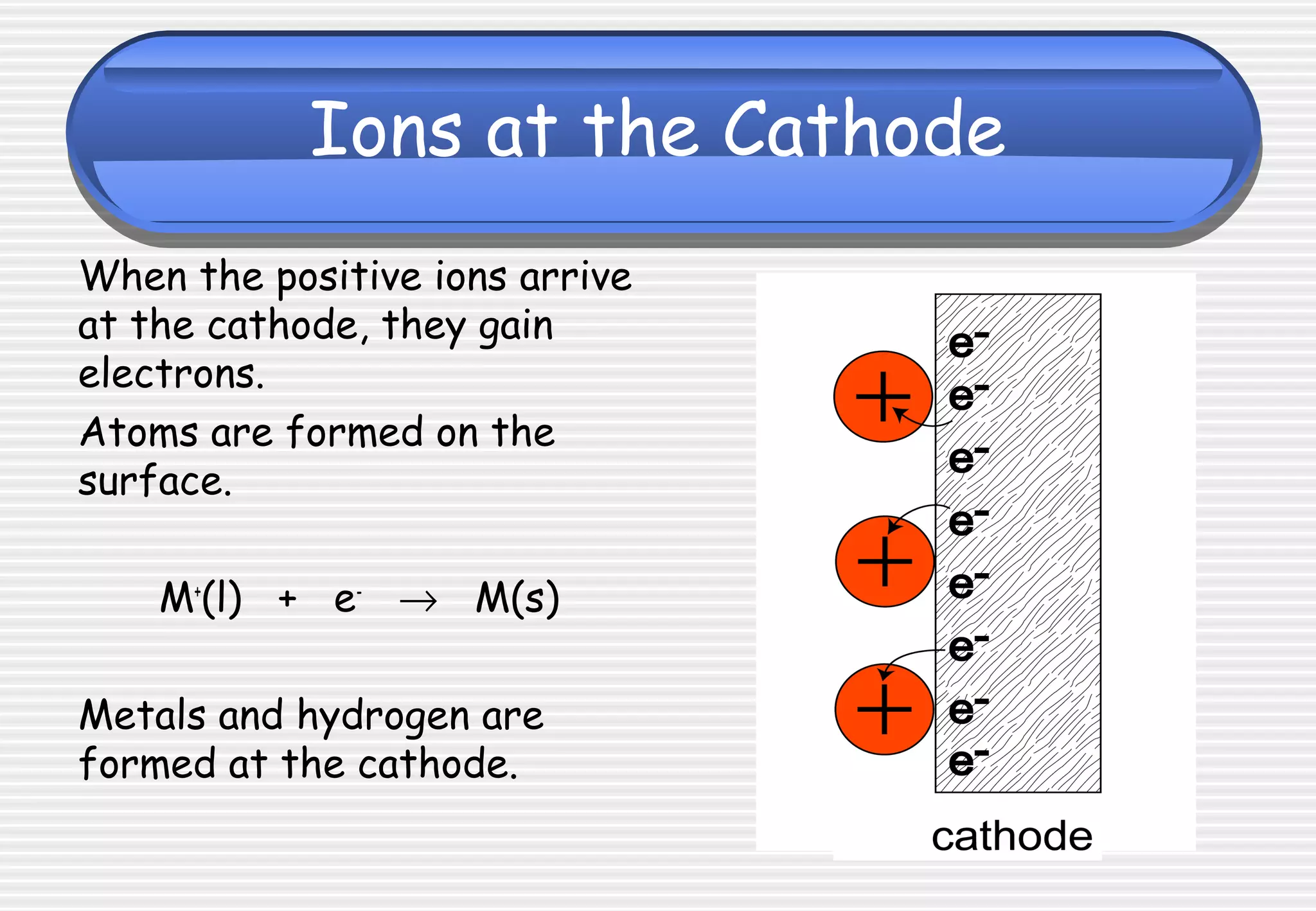
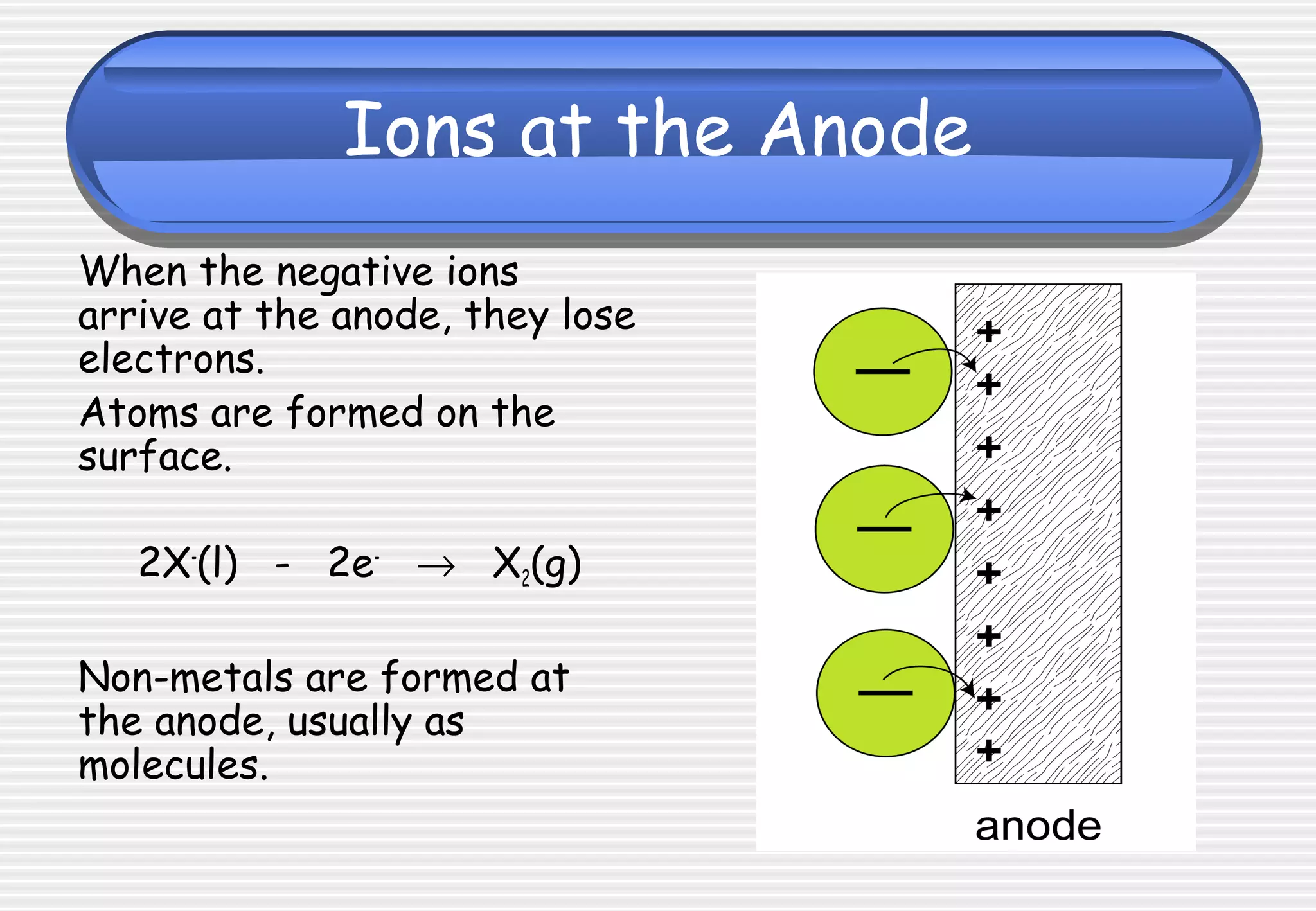
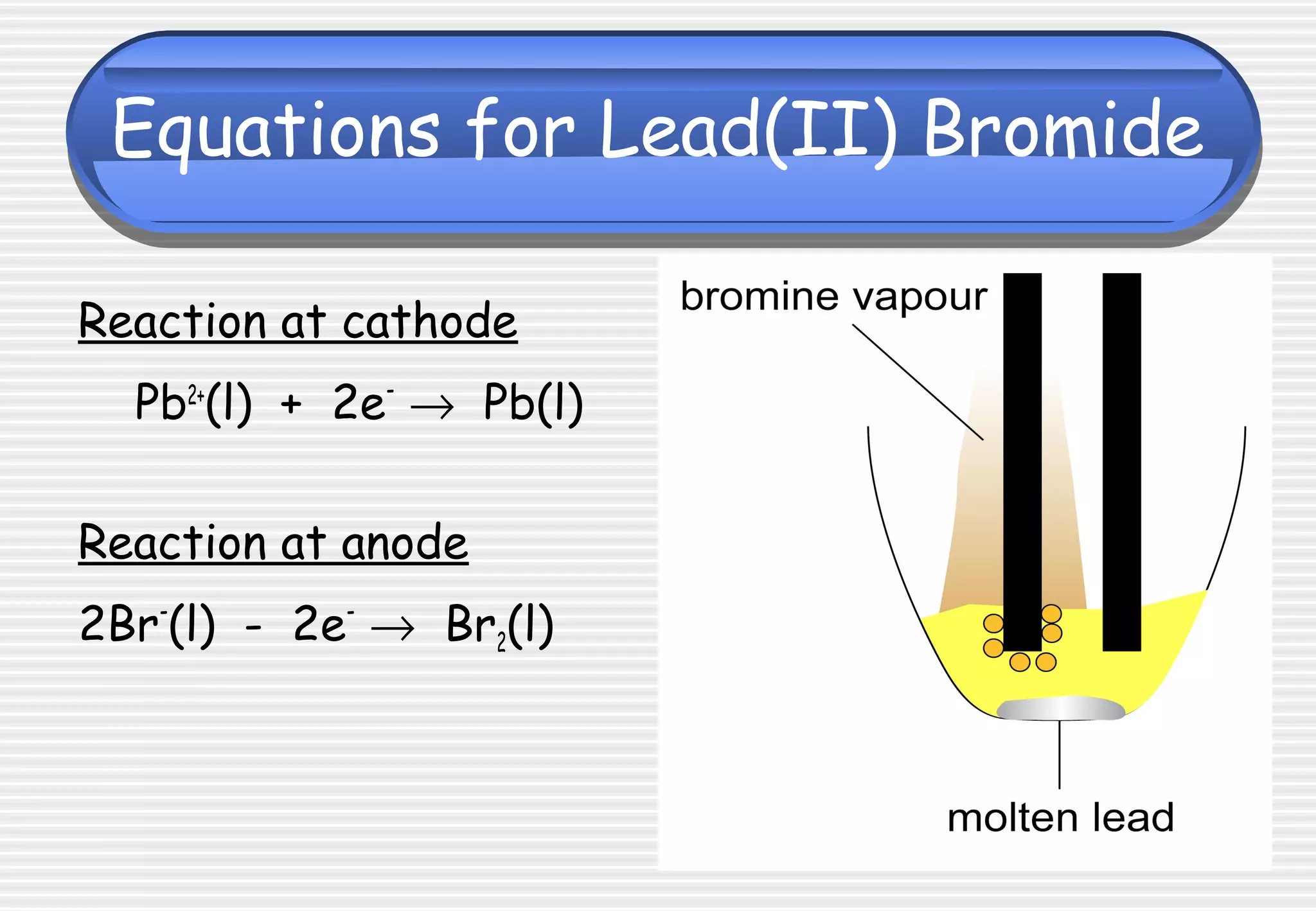
This document provides definitions and explanations related to electrolysis. It defines key terms like electrolyte, electrode, anode, and cathode. It explains that electrolysis involves the decomposition of ionic compounds through the movement of ions when a current is passed. Molten salts and those dissolved in solutions conduct electricity because the ions are free to move. Simple experiments are described to demonstrate electrolysis, including the electrolysis of molten lead(II) bromide. Equations are given for the reactions at the anode and cathode.