Embed presentation



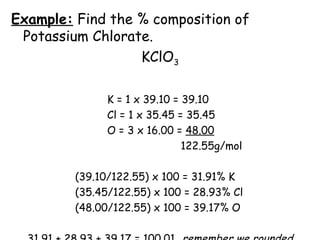



This document discusses how to calculate the percentage composition of compounds from their chemical formulas. It explains that the chemical formula tells the number of moles of each element in one mole of the compound. It then provides steps to calculate percentage composition: write the chemical formula, find the molar mass of the compound and each element, divide the molar mass of each element by the total molar mass and multiply by 100. Examples are given for potassium chlorate and ammonium phosphate.






