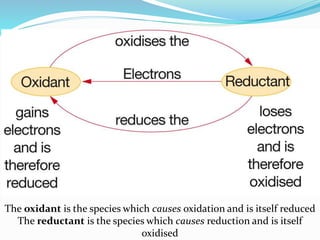
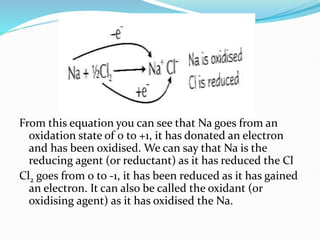
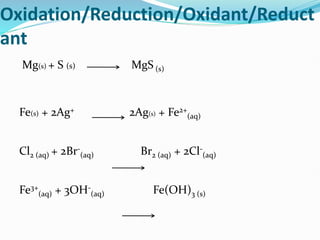
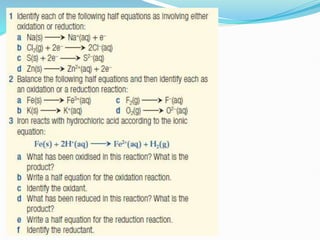
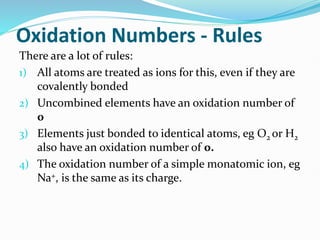
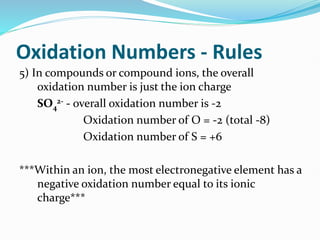
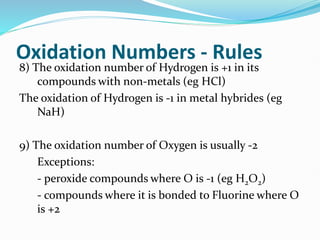
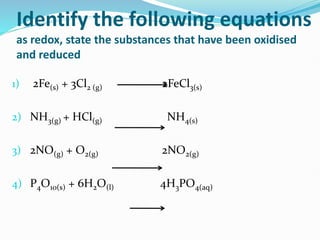
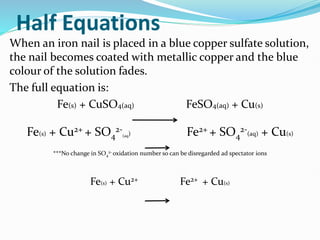
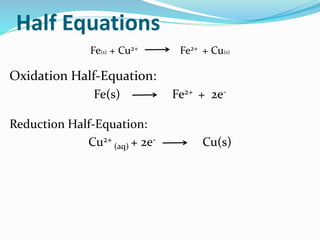
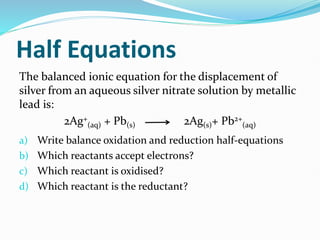
Redox reactions involve the transfer of electrons from one reactant to another, resulting in oxidation and reduction. Oxidation is the loss of electrons and reduction is the gain of electrons. Common redox reactions include photosynthesis, respiration, combustion, and production and use of fertilizers.