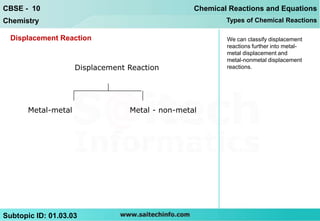
1) Displacement reactions can be classified as metal-metal displacement reactions or metal-nonmetal displacement reactions.
2) In metal-metal displacement reactions, more reactive metals displace less reactive metals according to the reactivity series. For example, copper displaces iron when an iron bar is placed in copper sulfate solution.
3) In metal-nonmetal displacement reactions, metals can displace hydrogen from water, with more reactive metals like sodium displacing hydrogen even at room temperature, while less reactive metals like iron only displacing hydrogen when heated.