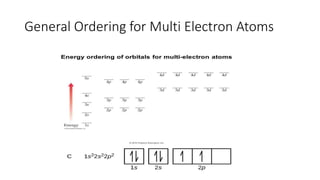
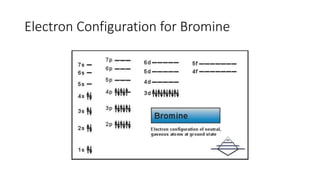
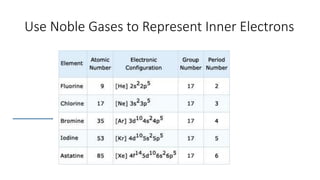
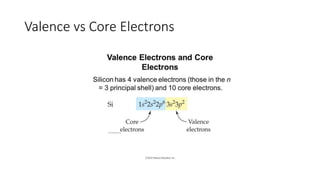
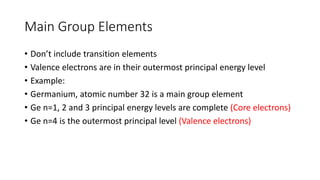
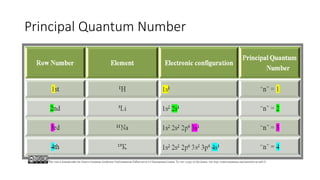
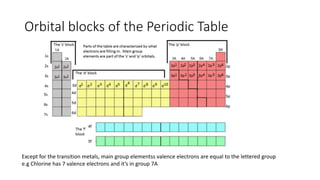
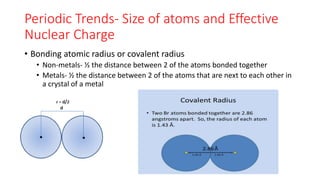
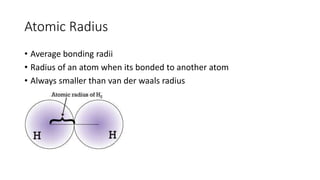
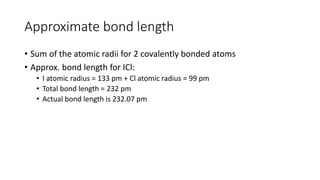
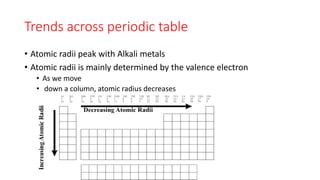
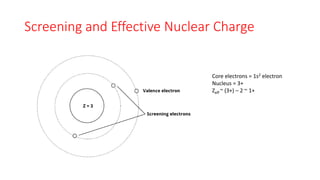
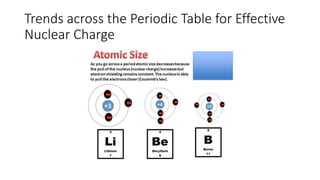
This document discusses electron configuration and chemical bonding. It begins by reviewing general ordering rules for electron configuration and defining core and valence electrons. It then discusses trends in atomic size and effective nuclear charge across the periodic table. As atomic number increases within a period, atomic radius generally decreases due to increased nuclear attraction. Transition metals are an exception, as their atomic radii remain relatively constant as valence electrons do not change.