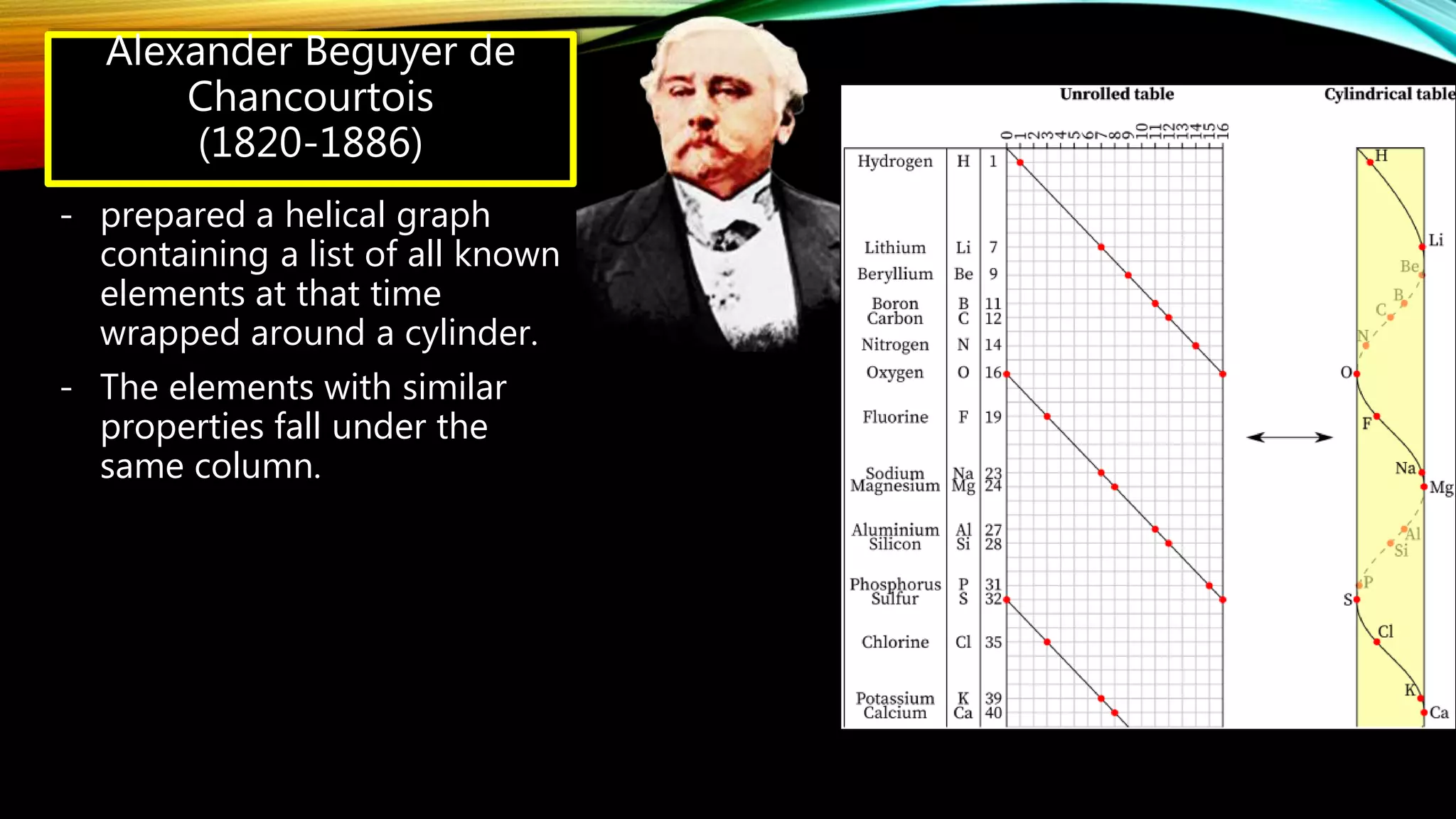
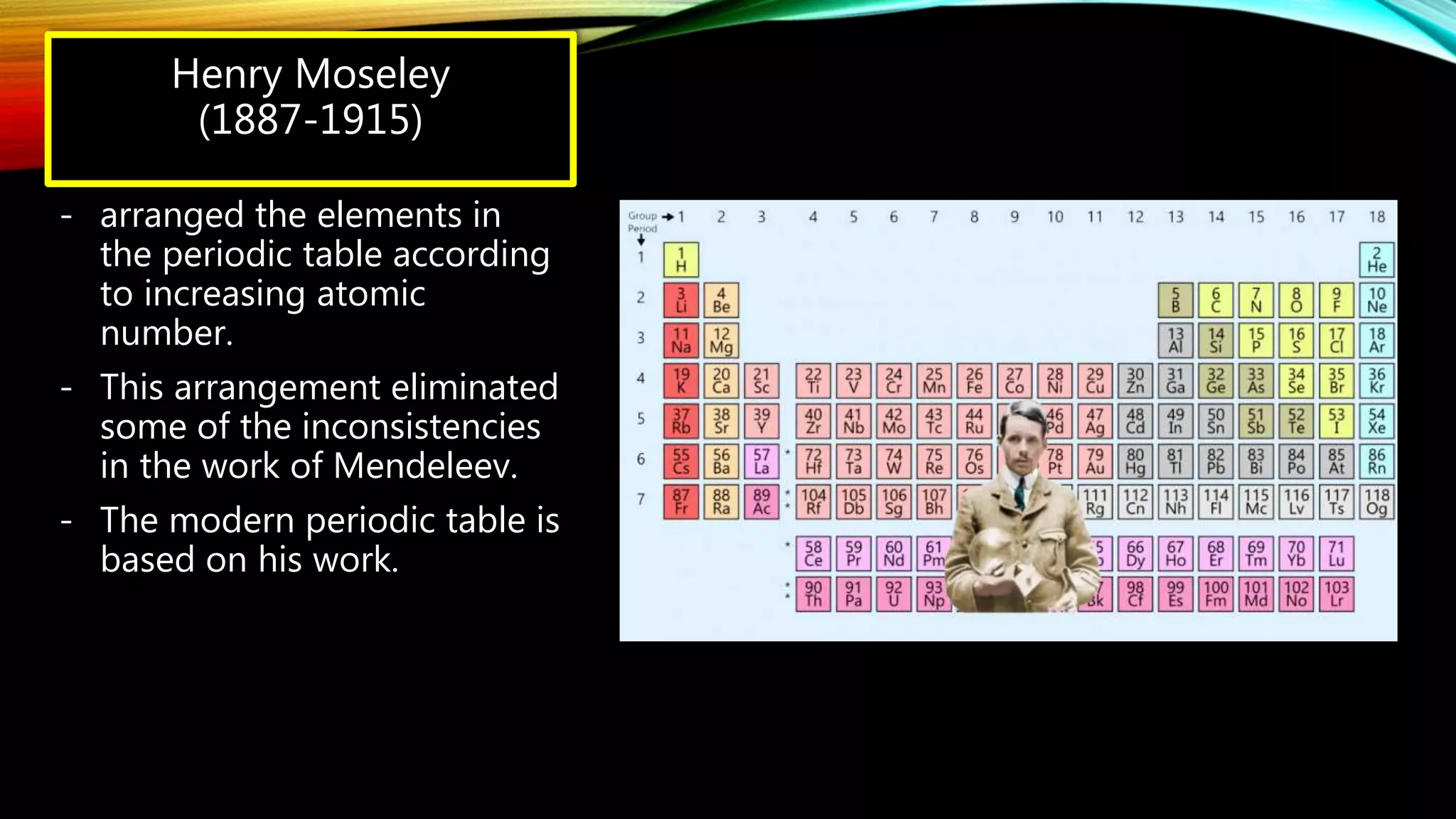
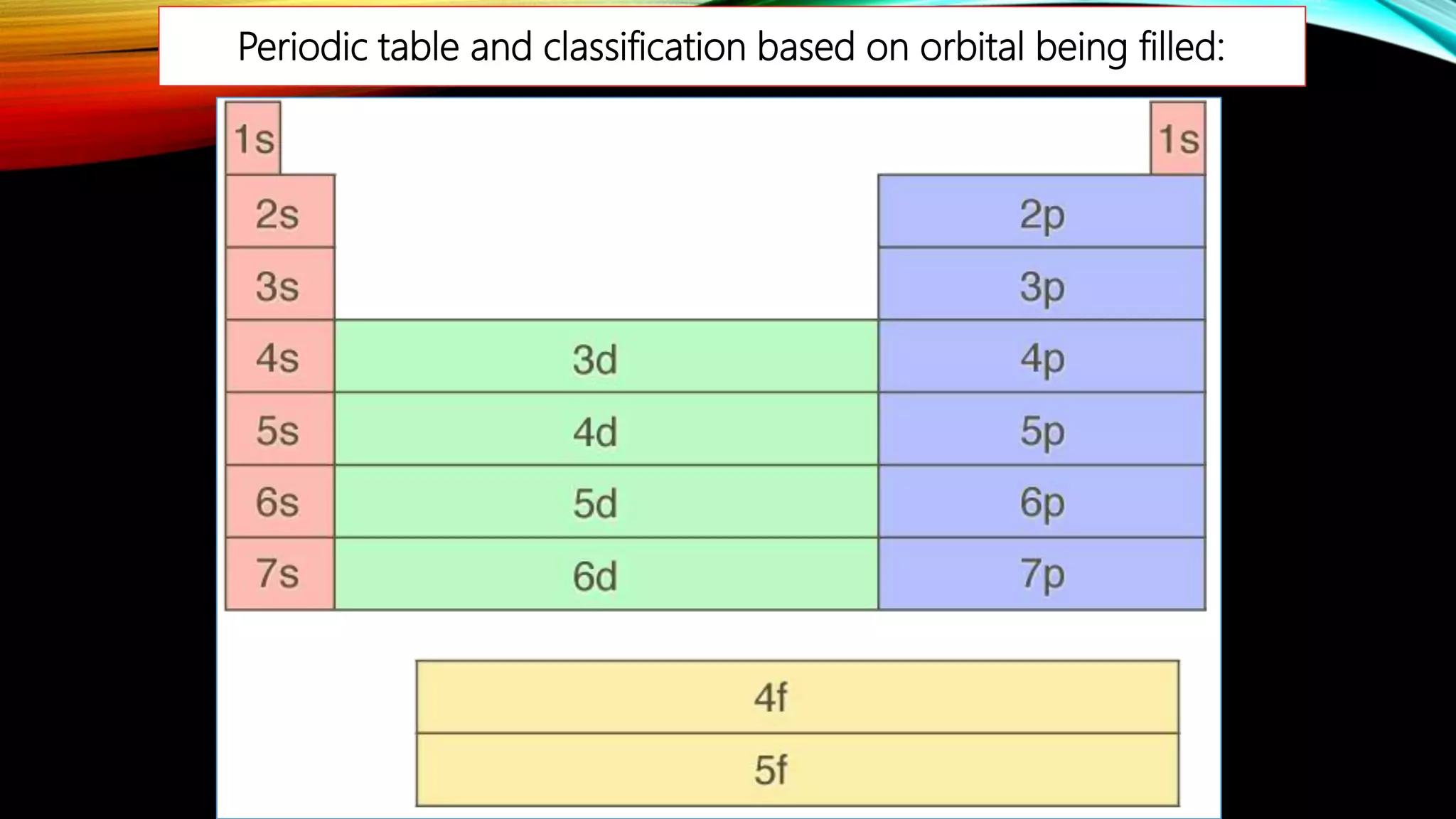
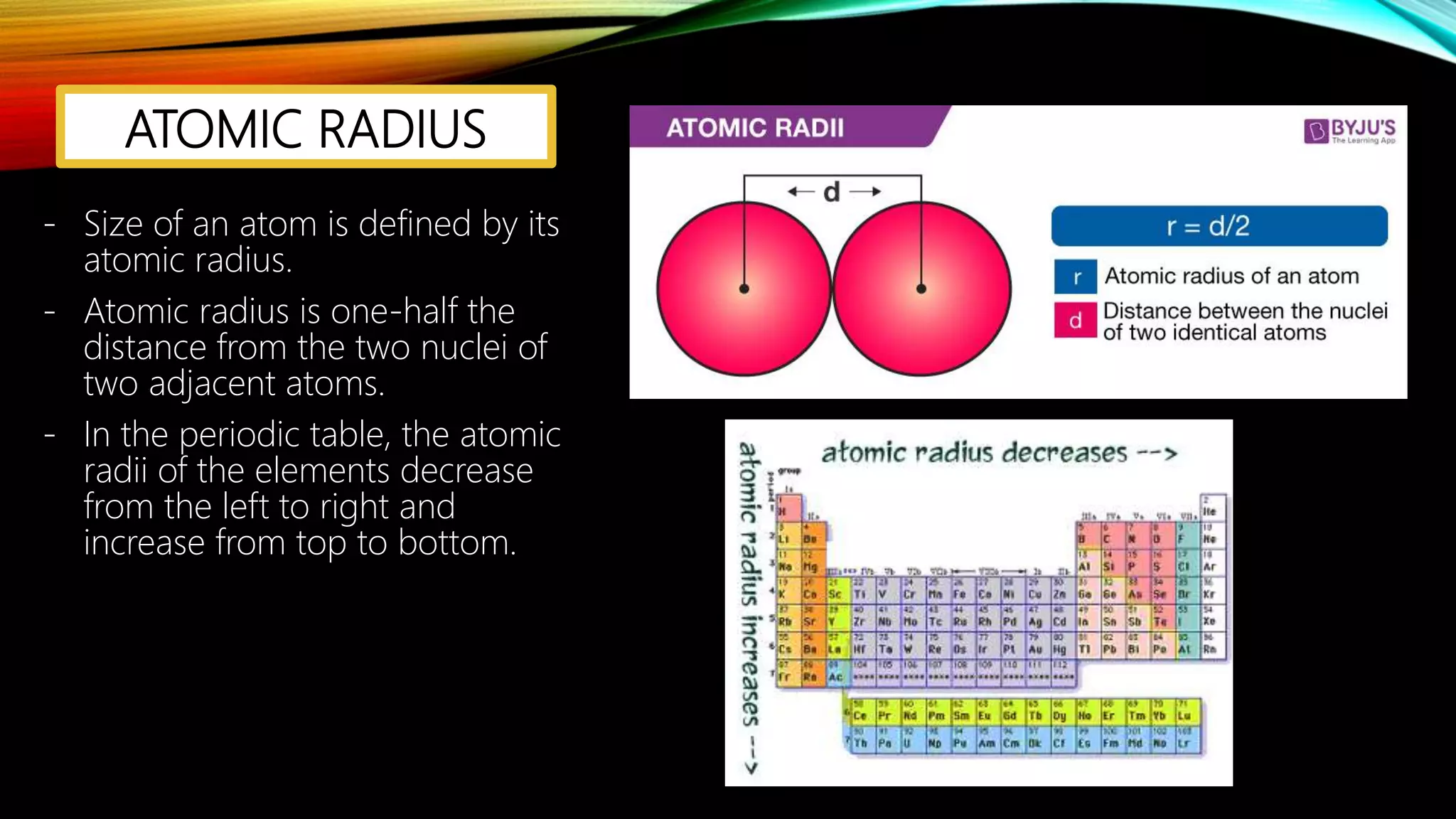
The document summarizes the key people involved in the discovery and development of the periodic table of elements. It discusses Johann Dobereiner who discovered triads of elements, de Chancourtois who arranged elements in a helix, Newlands who proposed periodicity based on atomic mass, and Mendeleev who created one of the first recognizable periodic tables. It also mentions the contributions of Meyer, Ramsay, Moseley, and Seaborg in refining the table and adding new elements. The periodic table organizes elements based on electron configuration in their outermost shells and exhibits trends in properties from atomic radius to metallic character across the table.