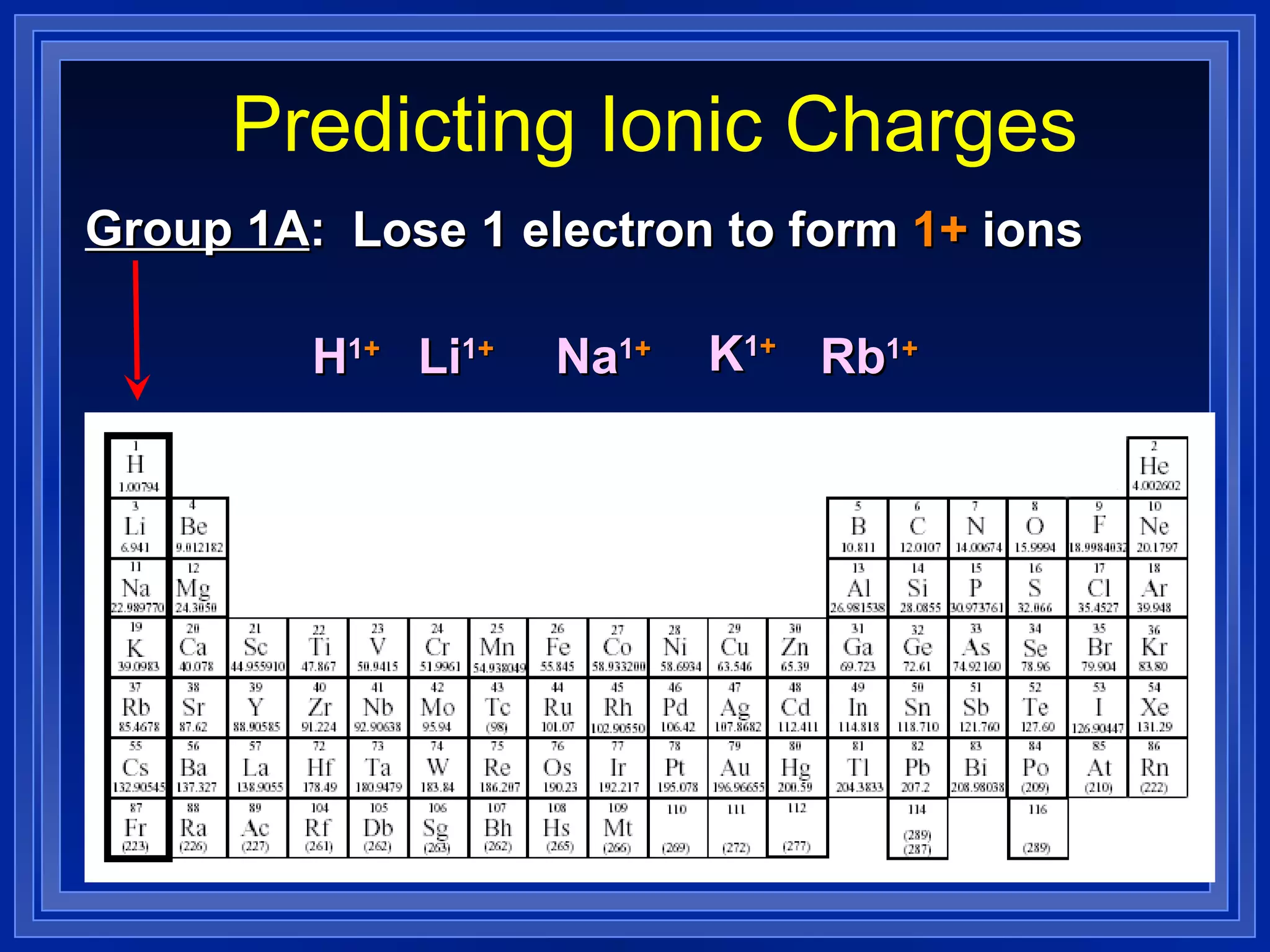
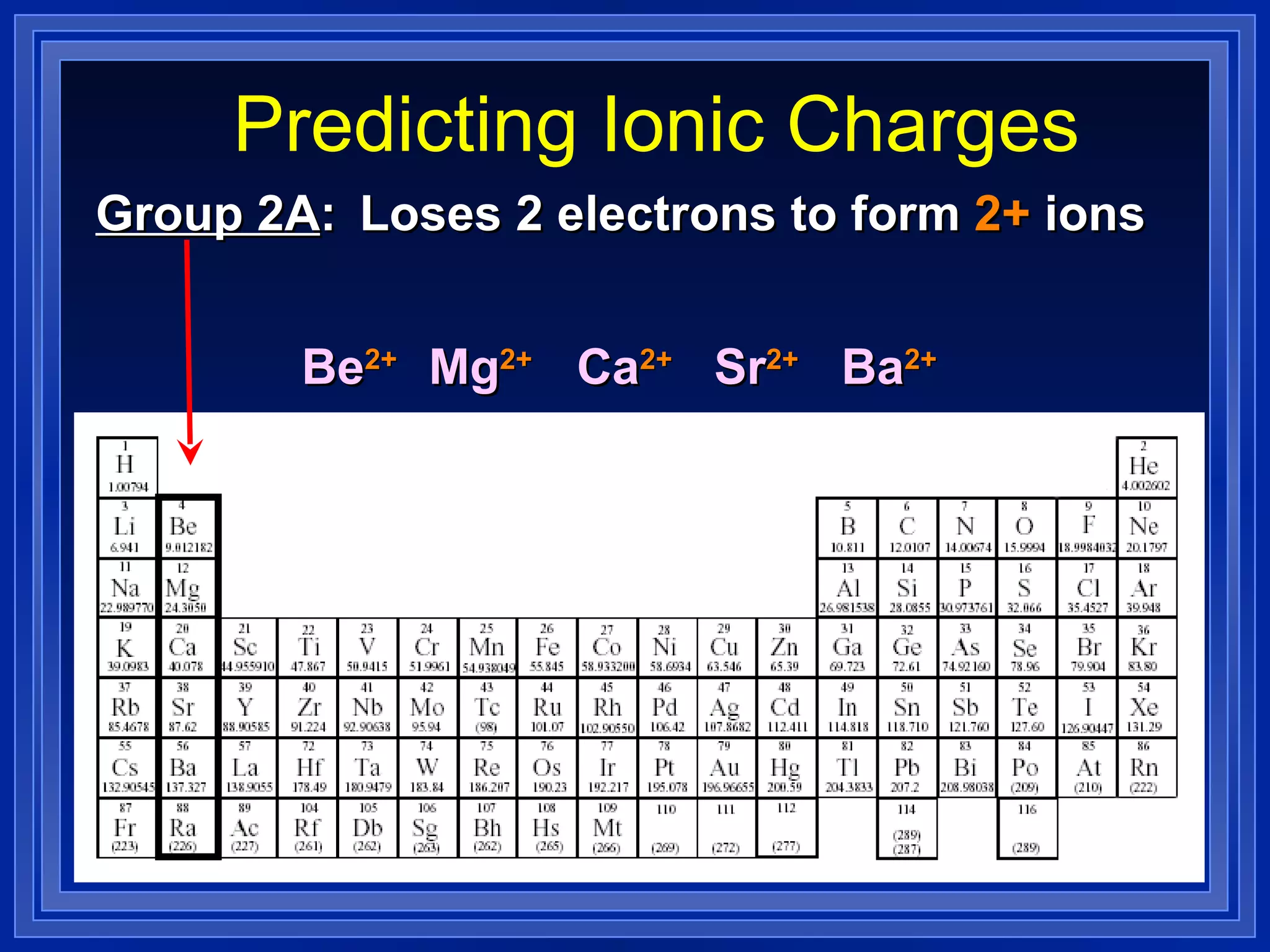
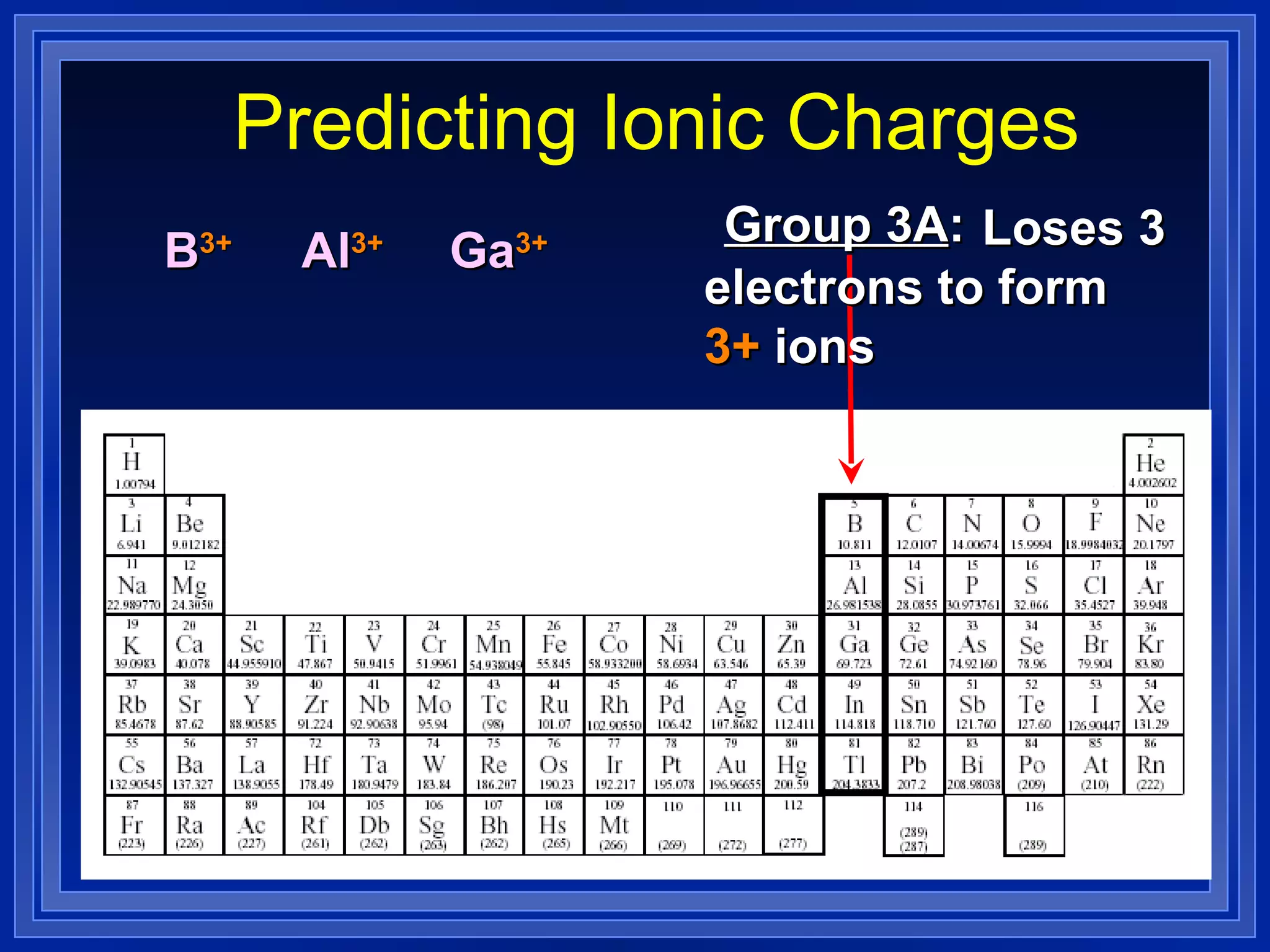
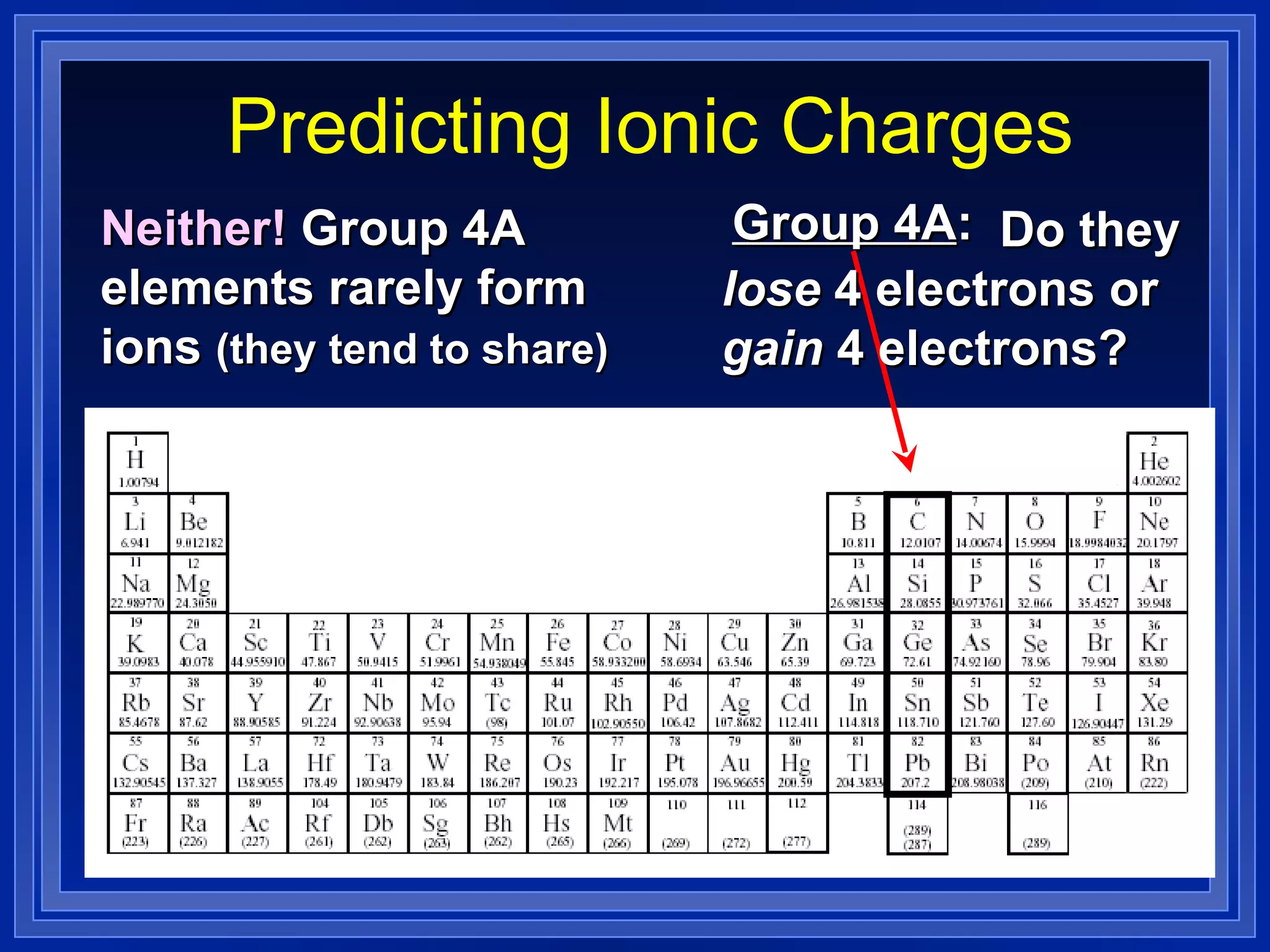
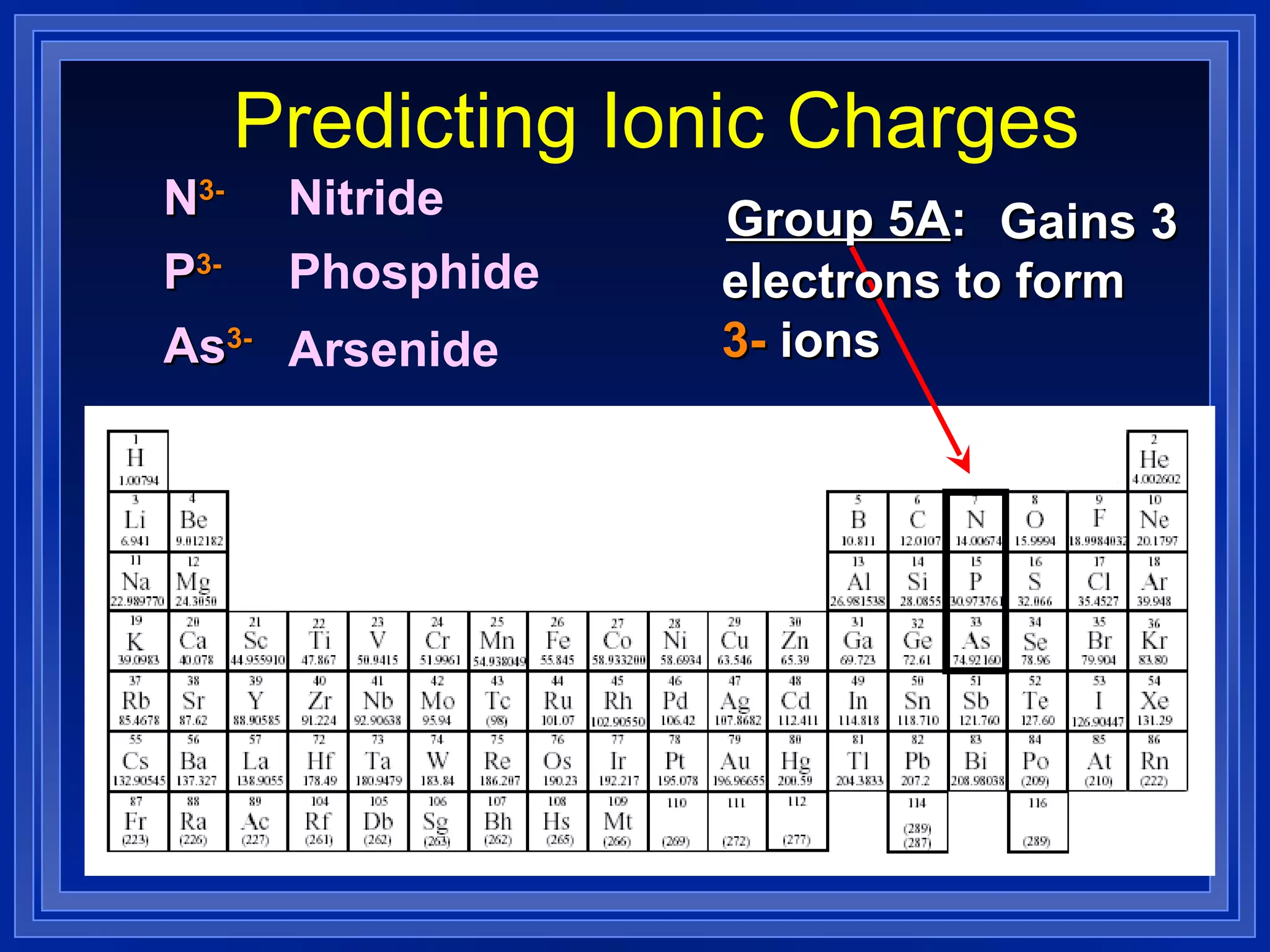
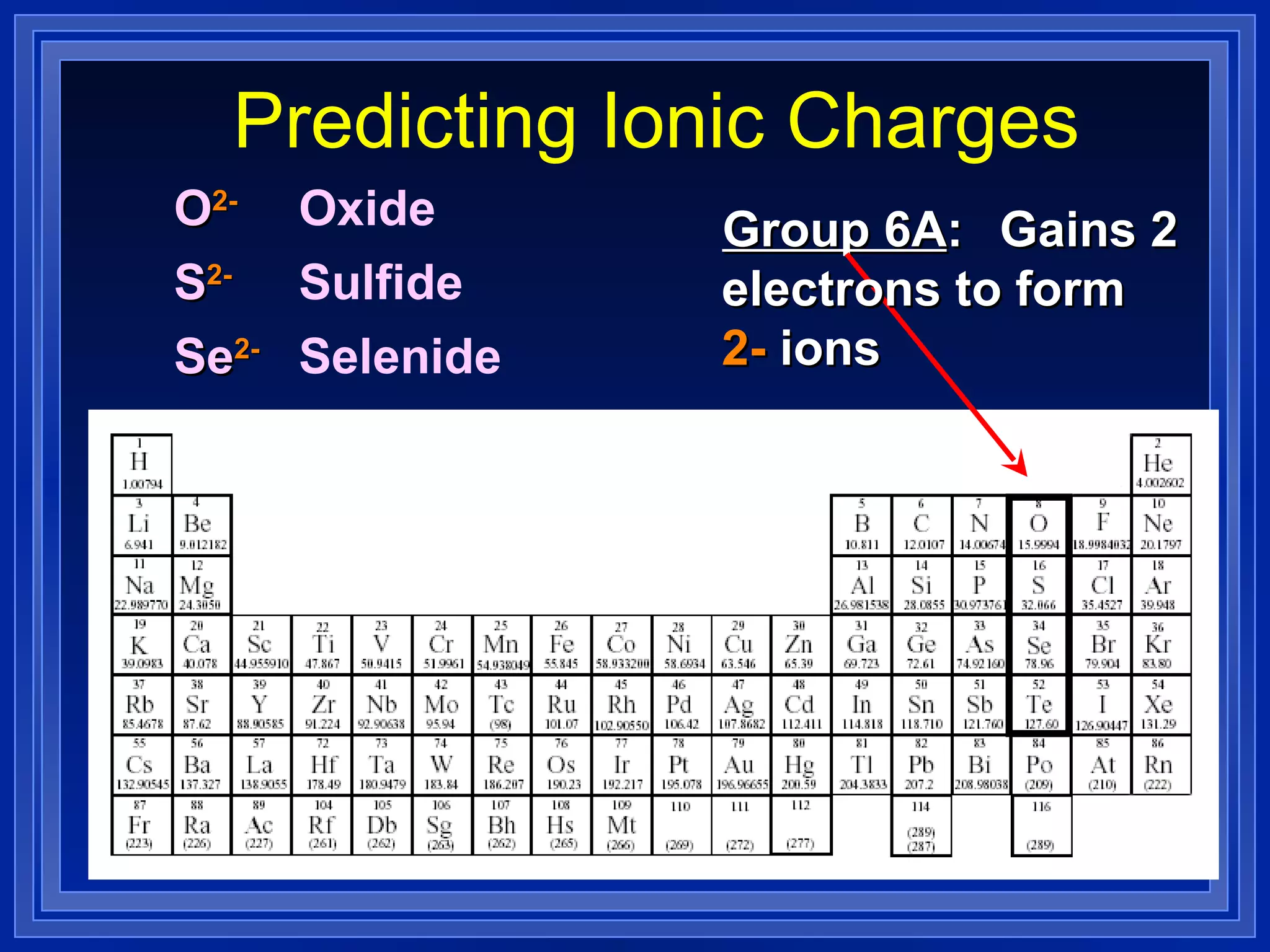
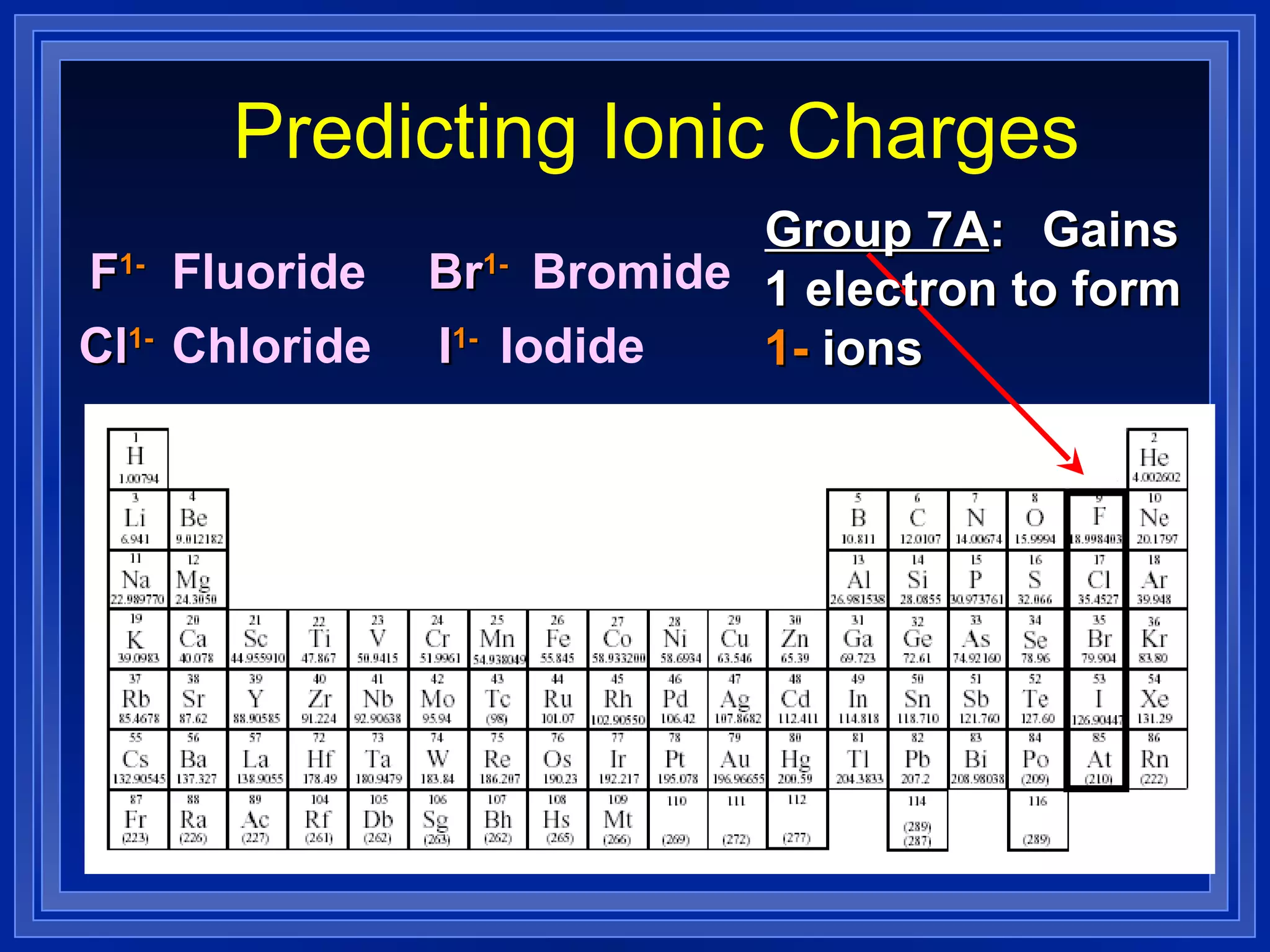
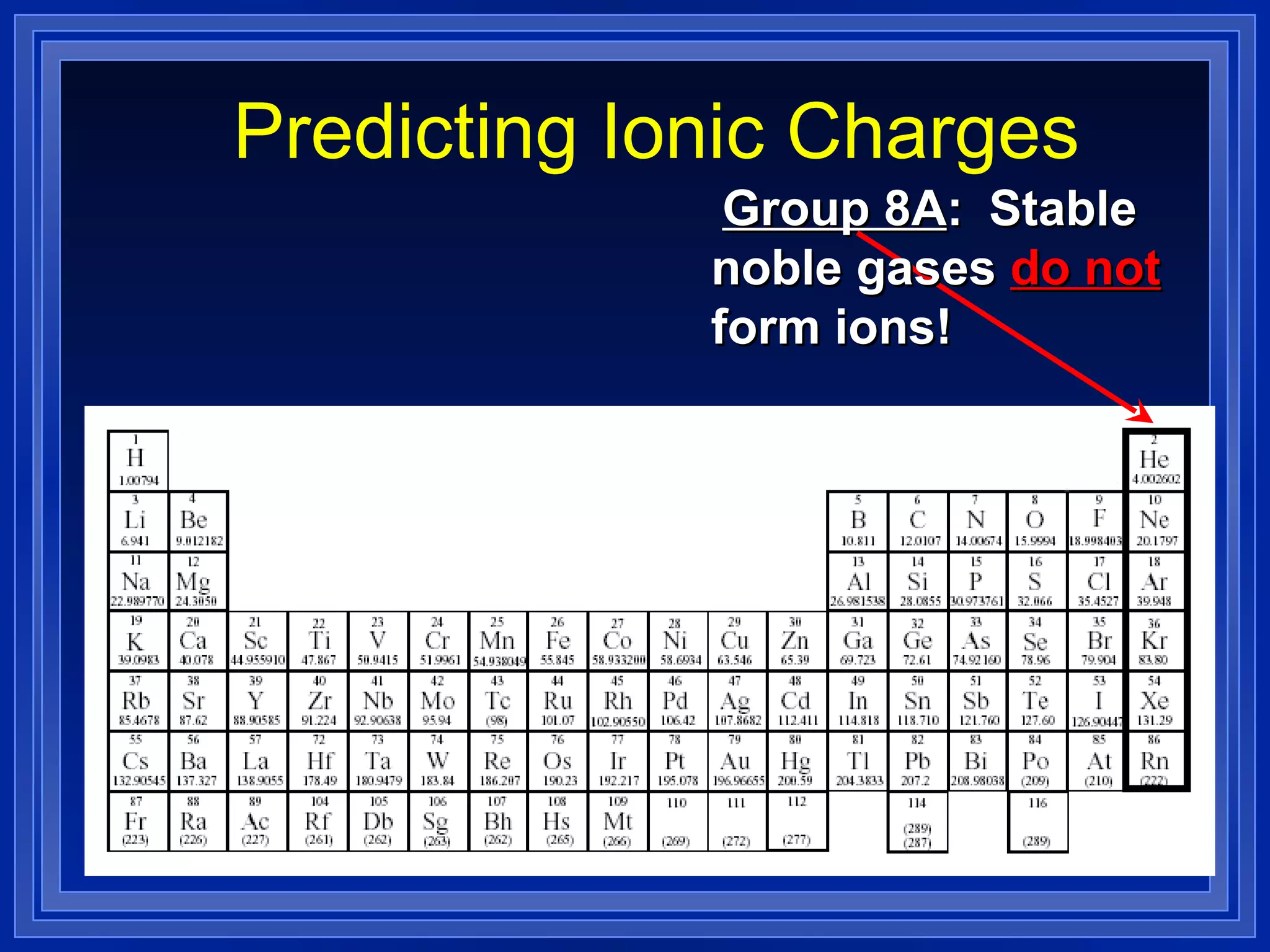
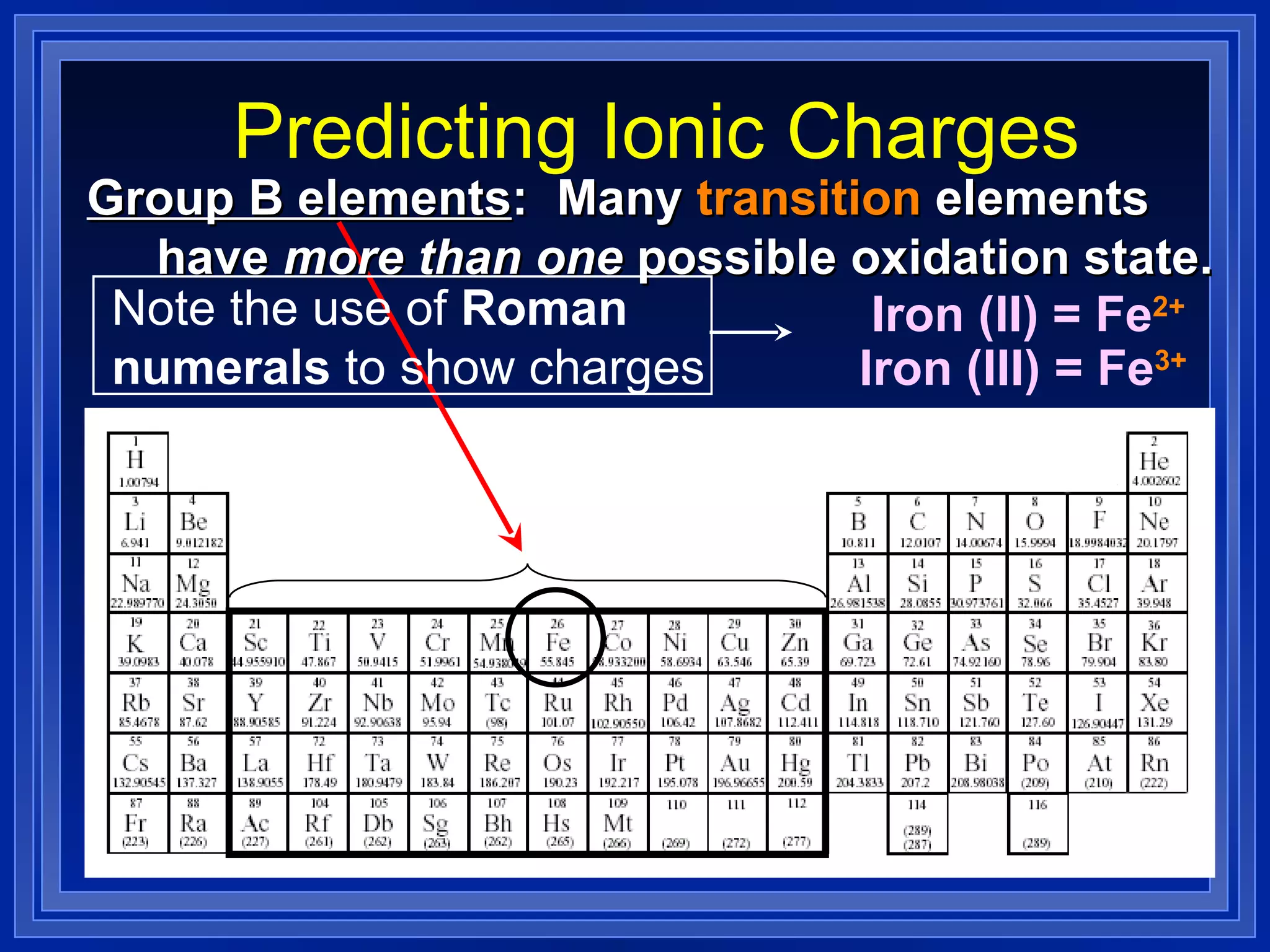
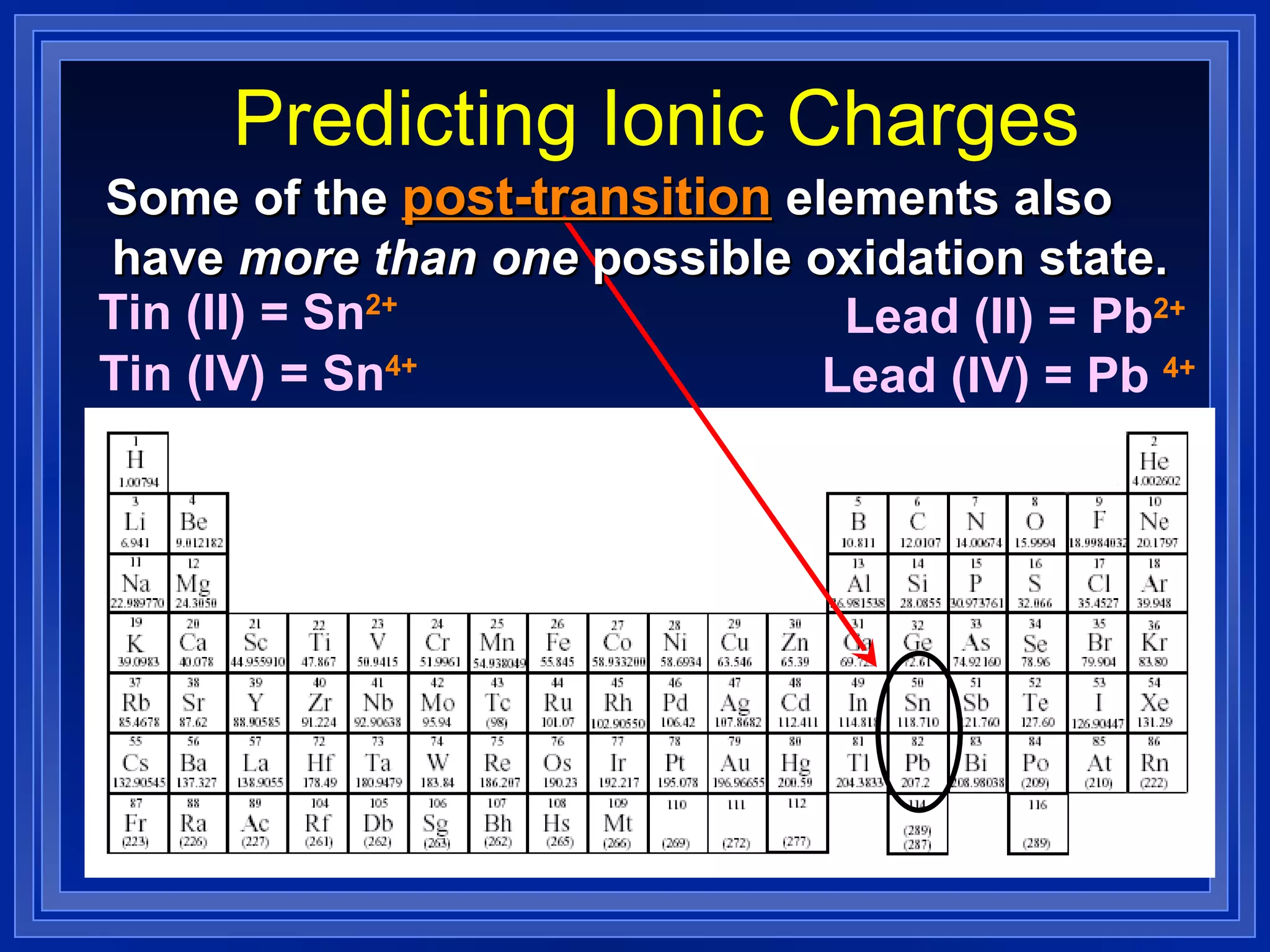
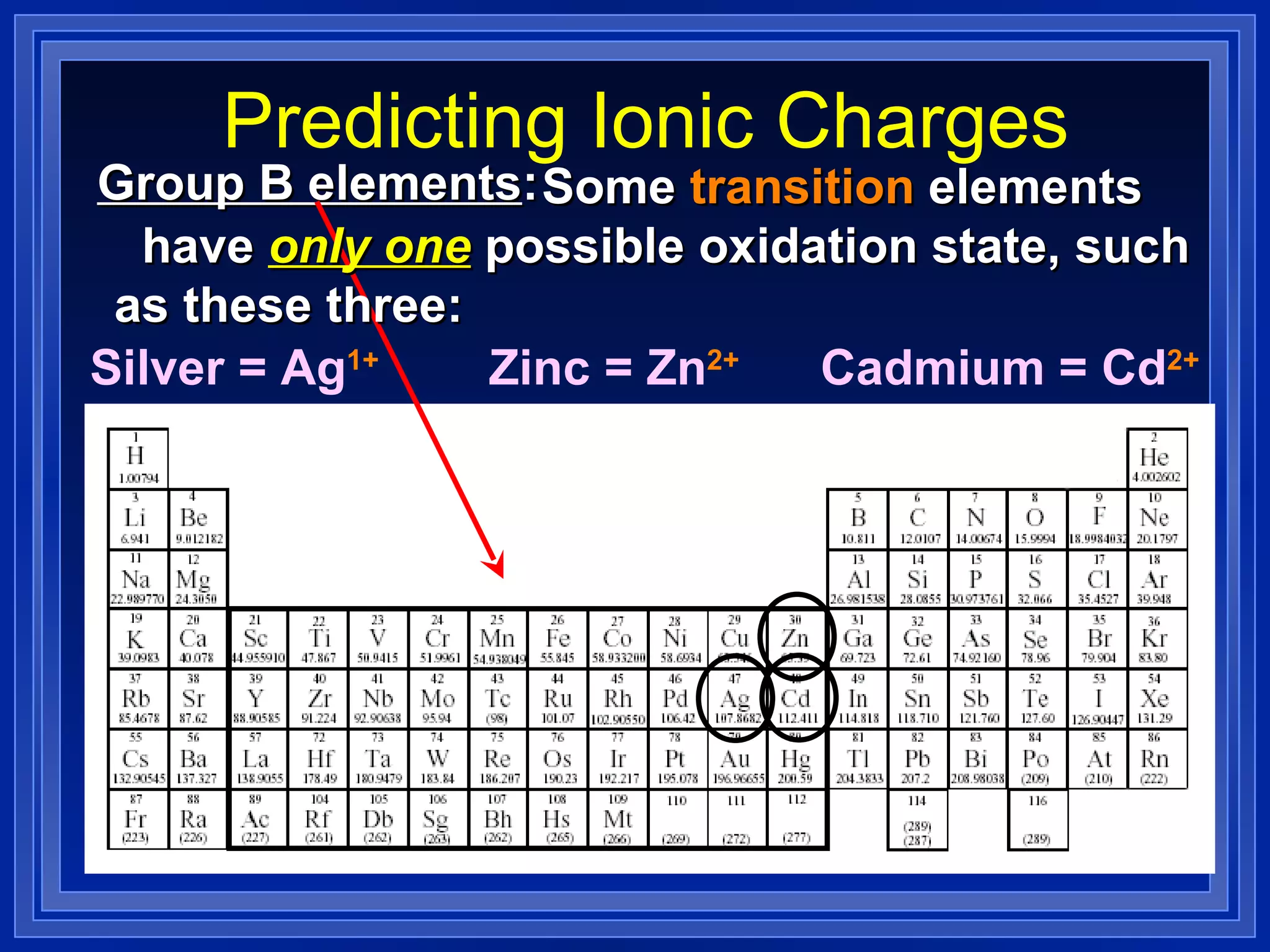
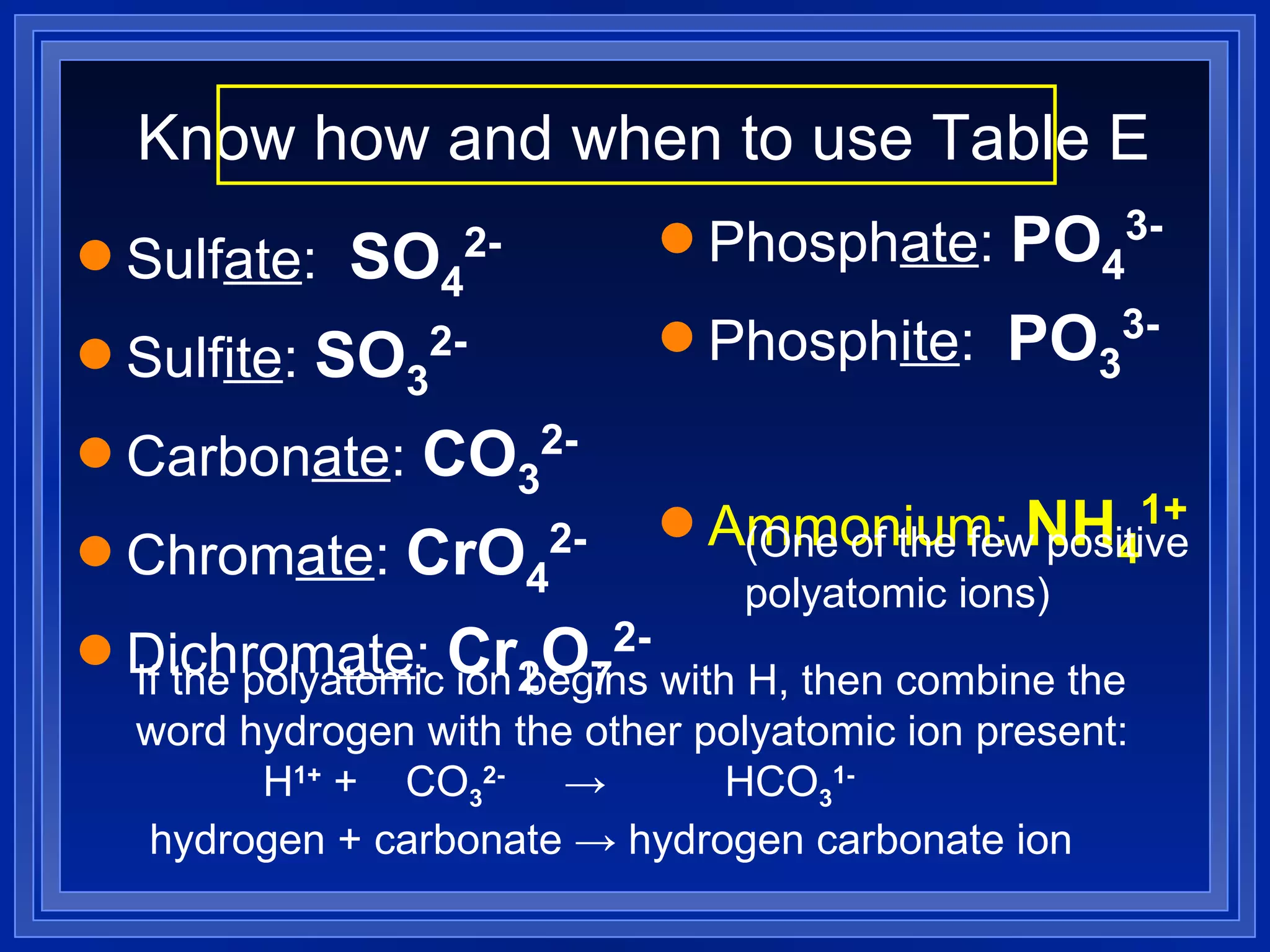
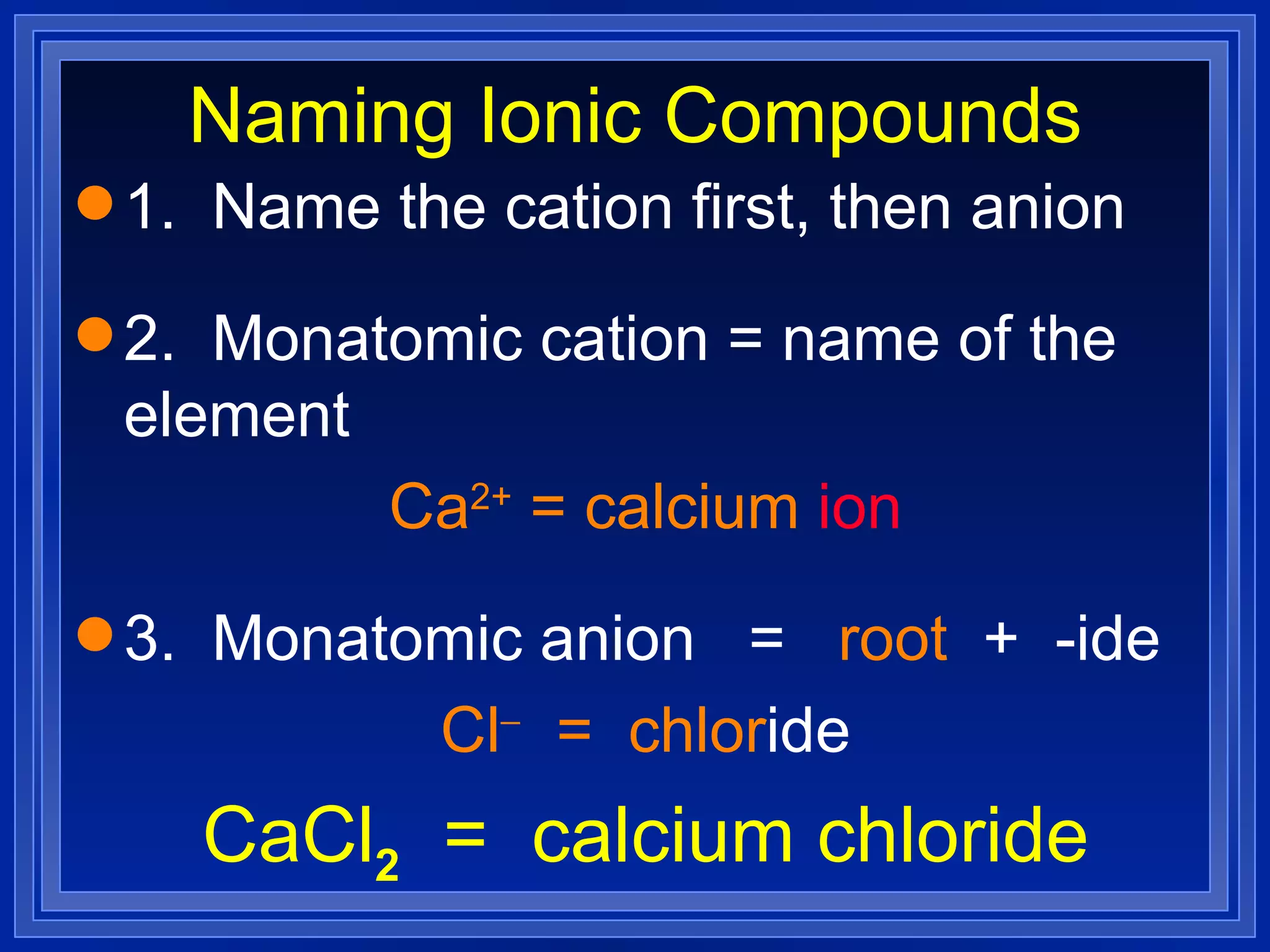
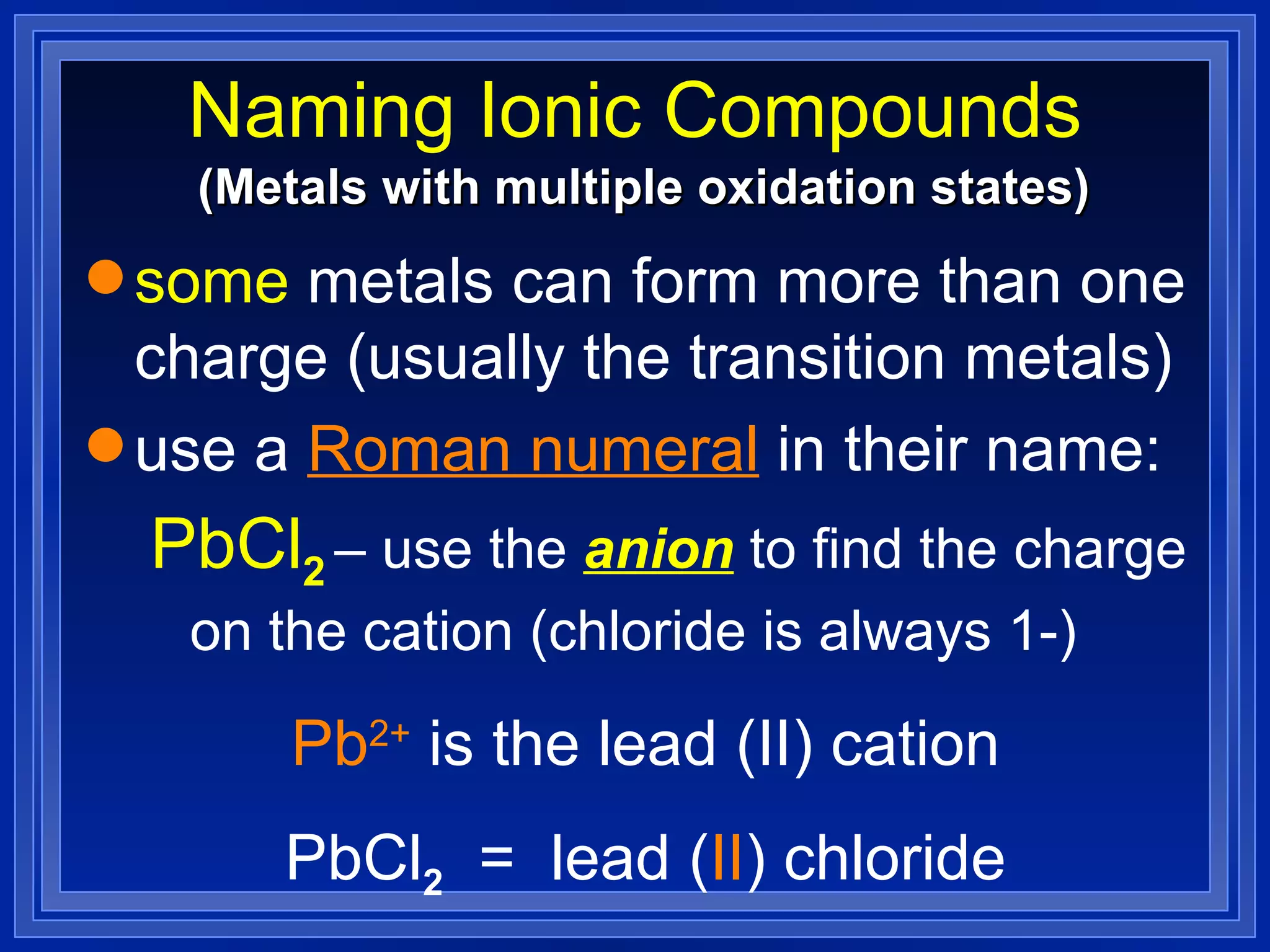
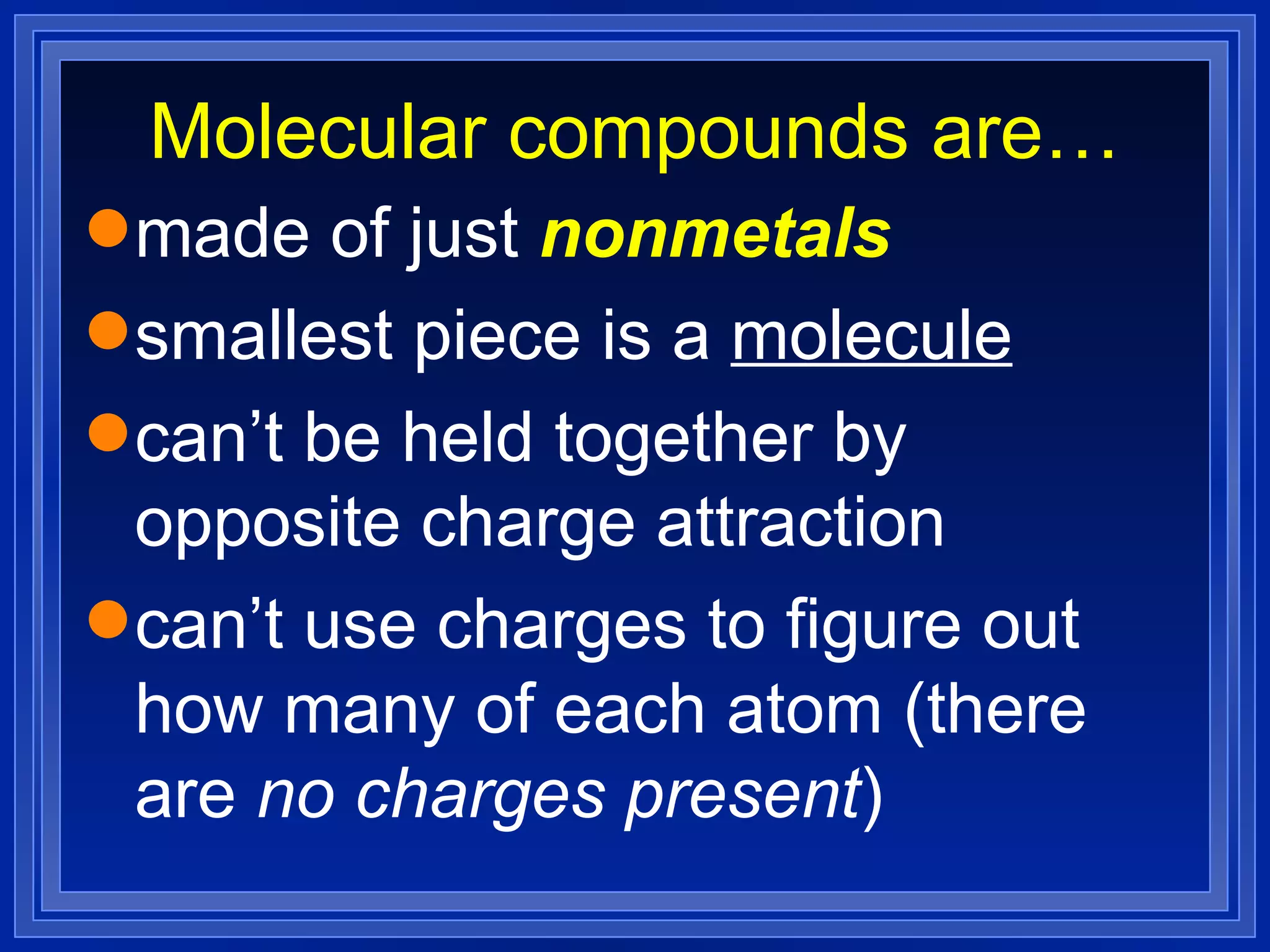
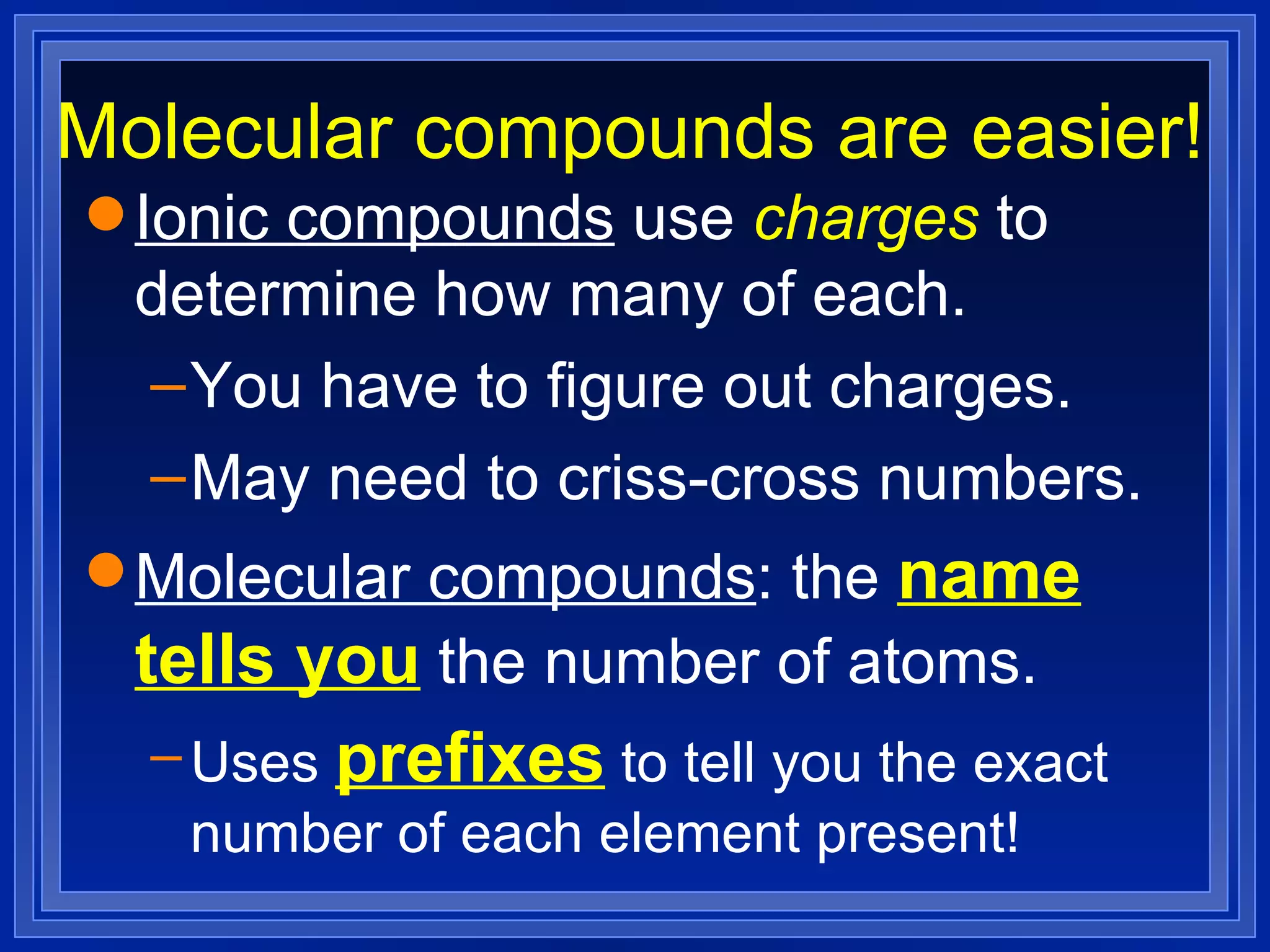
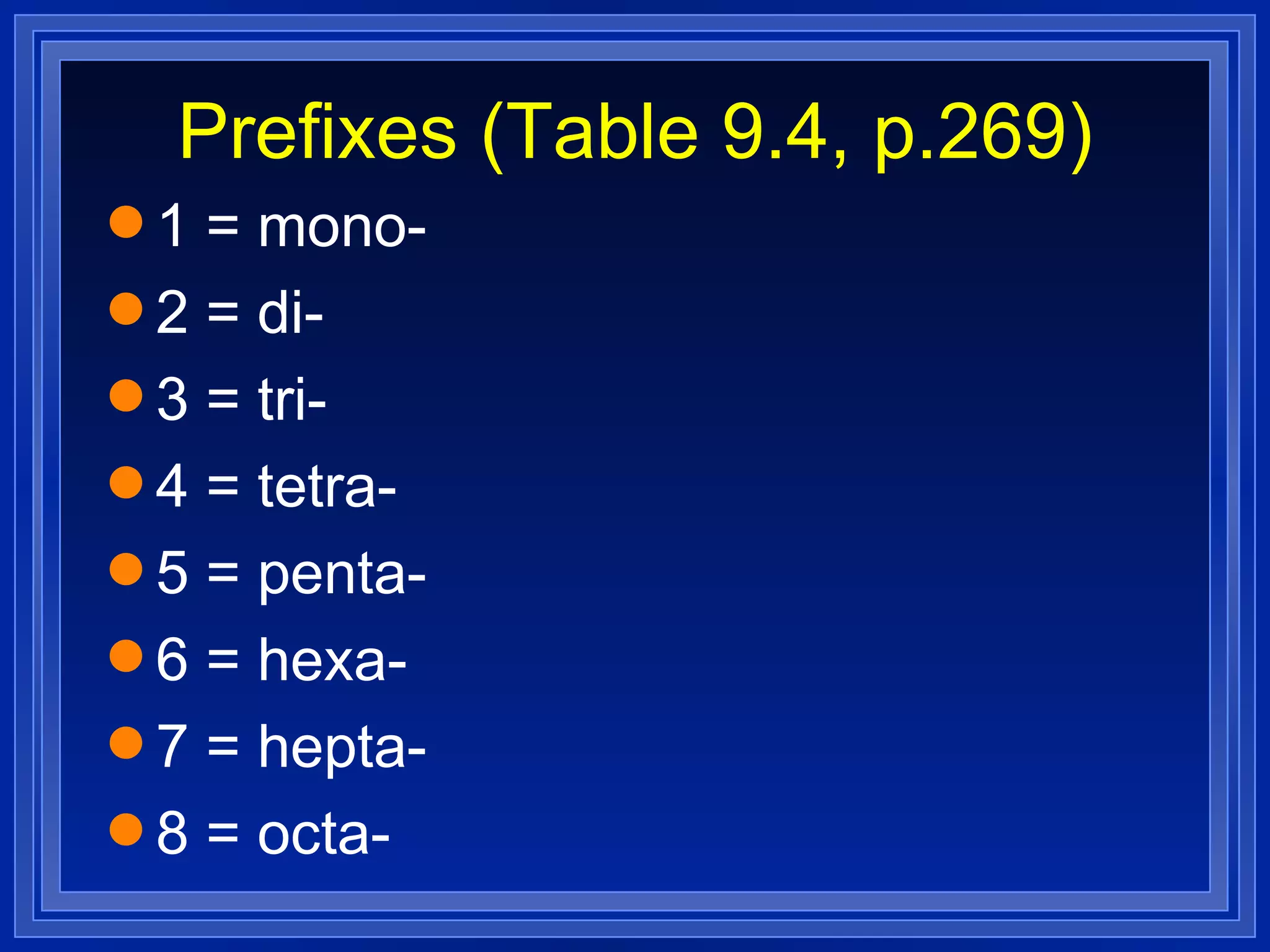
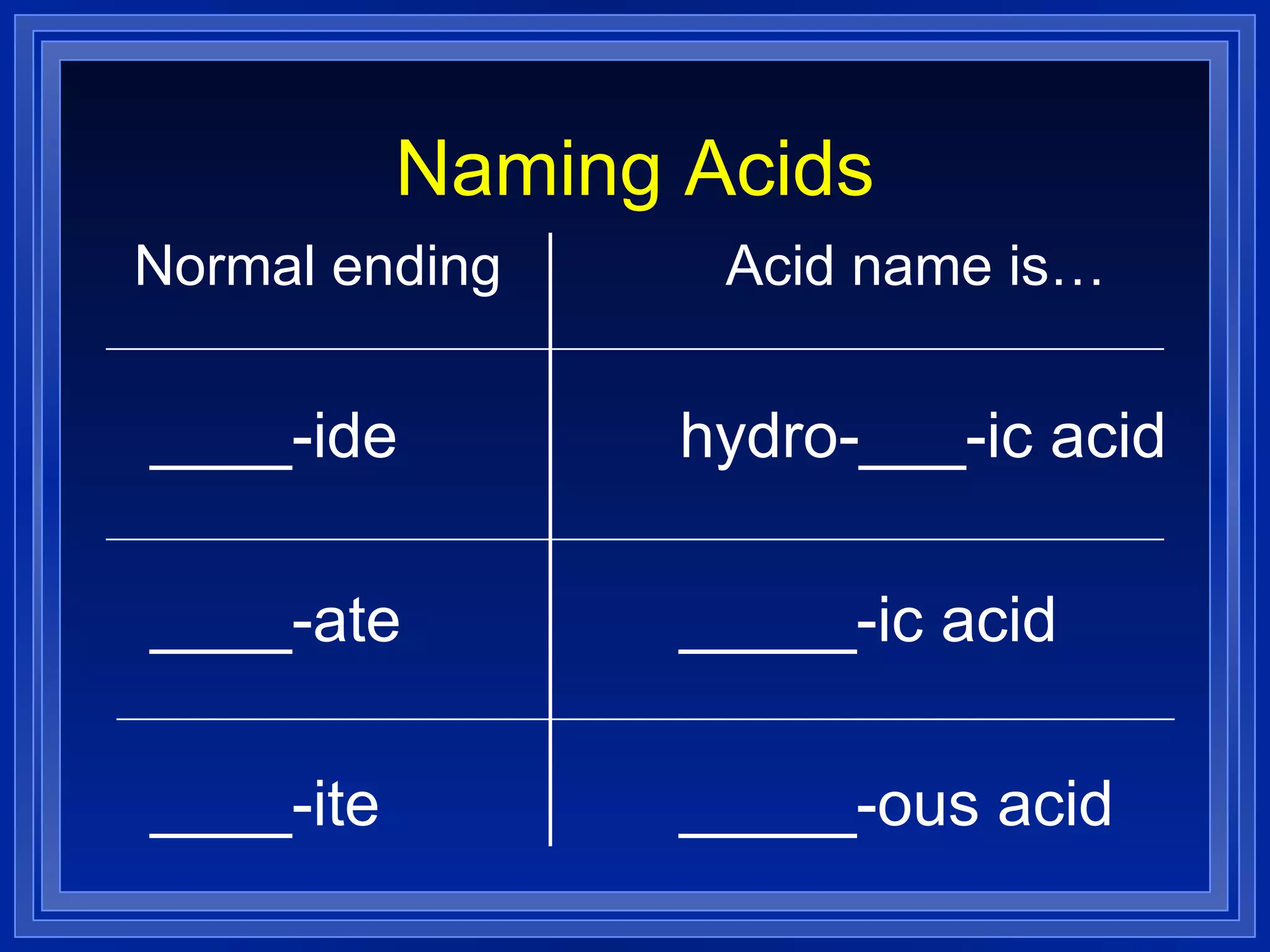
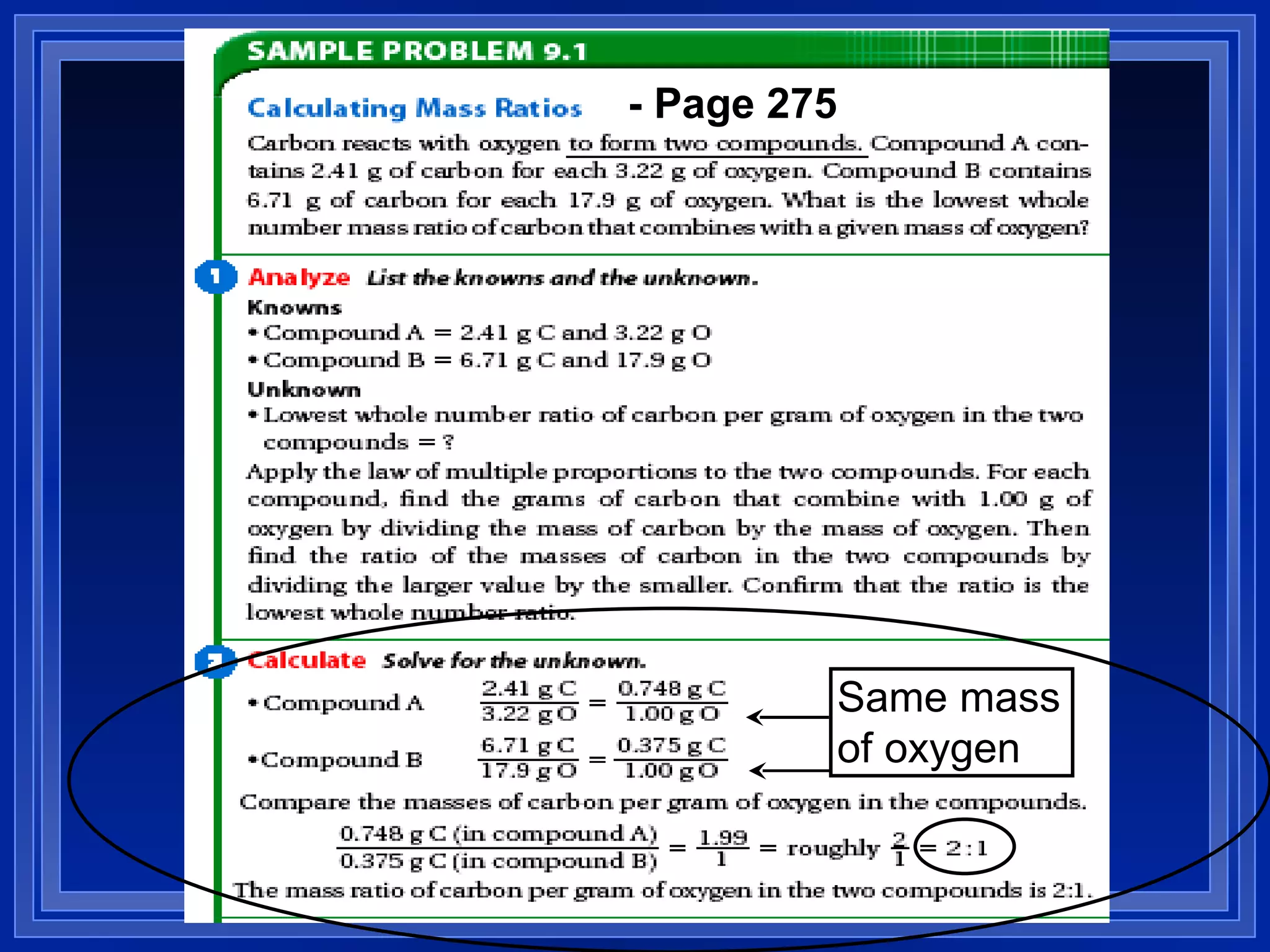
This document covers naming and writing formulas for ions, ionic compounds, molecular compounds, acids, and bases. It provides objectives and definitions for each section, examples of naming and writing formulas, and explanations of naming conventions and rules including prefixes, charges, and endings for different types of compounds.