Unit3presentation
- 1. Unit 3 Quantum Theory And Periodicity
- 2. Unit 3 - Quantum Theory and Periodicity The quantum mechanical model is the current model of the atom that describes the probability of finding an electron in a given region of space around the nucleus. It is called the electron cloud model .
- 4. Quantum numbers are the 4 numbers that specify the properties of atomic orbitals and the electrons that reside in them.
- 5. The energy level, also called the principal quantum number or shell, is the first quantum number. Electrons can occupy only specific energy levels. These levels are numbered 1 – 7 . The higher energy levels indicate a higher energy state for that electron and a location further away from the nucleus. In other words, electrons near the nucleus are low energy electrons.
- 7. The energy level indicates the size of the electron cloud. The formula 2n 2 indicates the total possible electrons in an energy level.
- 8. Example: Calculate the total possible electrons in the 1 st through 4 th energy levels. Remember: 2n 2 Energy Level # of Electrons 1 2 3 4
- 9. The orbital shape , also called the angular momentum quantum number , is the second quantum number. An energy level is actually made of many energy states called orbitals (subshells or sublevels).
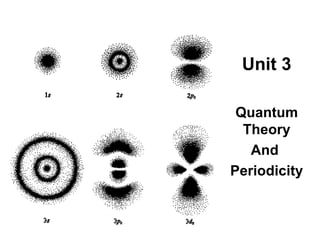
- 10. An orbital is a three dimensional region around the nucleus that indicates the probable location of one pair of electrons. These orbitals are s, p, d and f . The second quantum number indicates the shape of the orbital. S - orbital P - orbital D - orbitals
- 11. The s-orbital is sphere shaped.
- 12. The p-orbital is dumb bell shaped
- 13. d-orbitals look like leaves of clover
- 14. f-orbitals look like flowers
- 15. Orbital orientation , also called the magnetic quantum number , is the 3 rd quantum number. The magnetic number indicates the orientation of an orbital around the nucleus. Since an ‘s’ orbital is spherical , it can have only 1 orientation.
- 16. 3 Possible Orientations for p-Orbitals
- 17. d-Orbitals have 5 Possible Orientations
- 18. f-Orbitals have 7 Possible Orientations
- 19. Orbital Orientations 14 7 2 f 10 5 2 d 6 3 2 p 2 1 2 s Max # of Electrons if Each Orbital Orientation is Filled Possible Orientations for Each Type of Orbital MAX # Electrons per Orbital Orbital Type
- 20. The spin quantum number is the 4 th quantum number. The spin quantum number indicates that the 2 electrons occupying a single orbital must have opposite spin.
- 21. 32 2 6 10 14 16 1 3 5 7 s p d f 4 18 2 6 10 9 1 3 5 s p d 3 8 2 6 4 1 3 s p 2 2 2 1 1 s 1 Max # of Electrons per Main Energy Level (2n 2 ) Max # Electrons In Filled Orbitals # of Orbitals per Main Energy Level (n 2 ) # of Orientations per Orbital Type Types of Orbitals in Main Energy Level (n) Principle Quantum # Main Energy Level (n)
- 22. 2(n – 5) 2 8 2 6 (n – 5) 2 4 1 3 s p 7 2(n – 3) 2 18 2 6 10 (n - 3) 2 9 1 3 5 s p d 6 2(n -1) 2 32 2 6 10 14 (n - 1) 2 16 1 3 5 7 s p d f 5 Max # of electrons per Main Energy Level Max # of Electron per Filled Orbital # of Orbitals Per Main Energy Level # Of Orientations per Orbital Type Types of Orbitals in Main Energy Level (n) Principle Quantum Number: Main Energy Level (n)
- 23. The row numbers on the periodic table are the same as the principle quantum numbers or energy levels (n).
- 24. Energy levels overlap so the diagram shows the order of the sublevels. The Aufbau principle states that an electron occupies the lowest energy orbital that can receive it.
- 25. Writing Electron Configurations Using the atomic number as the total number of electrons you can write the electron configuration for all of the elements. The number of electrons in each sublevel is written as a exponent .
- 26. Write the electron configuration for magnesium #12 gallium #31, element #35.
- 27. Periodic Table Orbital Filling Method
- 28. Use the periodic table to write the electron configurations for the following atoms. Example 1: Nitrogen, 7 electrons Example 2: Phosphorus, 15 electrons Example 3: Cerium, 58 electrons
- 29. Noble – Gas Notation The Group VIII elements, helium, argon, krypton, xenon, and radon are called the noble gases. The configurations of the noble gases are often used as a shorthand method for writing longer electron configurations. For Example: Sodium – Na has 11 electrons Electron Configuration 1s 2 2s 2 2p 6 3s 1 Noble – Gas Configuration [Ne]1s 2
- 30. Example 2: Arsenic, As, 33 electrons Electron Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3 Noble – Gas Configuration [Ar]4s 2 3d 10 4p 3 Example 3: Barium, Ba, 56 electrons Example 4: Rubidium, Rb
- 31. Using the electron configuration, you can find the valence electrons for that atom. Valence electrons are the electrons in the highest energy level.
- 33. Electron Dot Diagrams Lewis or electron dot diagrams are diagrams showing only the valence electrons in an atom. The diagram consists of the element symbol with as many as two dots on each of the four sides of the symbol. Electron configuration 1s 2 2s 2 2p 6 3s 2 3p 4
- 34. What is the electron configuration for Silicon? Write the electron dot diagram for silicon.
- 35. Write the electron configuration and electron dot diagram for phosphorus – atomic # = 15 and for Arsenic – atomic # = 33.
- 36. Electron – dot Periodic Table
- 37. LIGHT The ground state is the lowest energy level that an electron can occupy.
- 38. The excited state is a higher energy level that an electron may move to after absorbing energy .
- 39. The amount of energy absorbed by the electron is equal to the energy of the photon which is emitted.
- 40. A quantum leap is the jump in energy level that an electron will make after absorbing the correct quanta of energy. A quantum is a packet of energy that electrons absorb to change energy levels.
- 41. Light (photons) is the form of some of the electromagnetic radiation (energy) released by electrons as they return to their ground state from their excited state.
- 42. The particular wavelength of light produced is specific for each element and can be used to identify it.
- 43. Electromagnetic radiation is a form of energy that exhibits wavelike behavior as it travels through space.
- 44. A spectroscope is a device that is used to view the visible wavelengths of light produced by different atoms. The wavelengths are visible as bright lines on the spectrum.
- 45. A flame test is a method used to identify and element by the color of flame it produces. For example, copper produces a characteristic green flame.
- 47. Dimitri Mendeleev He develop the 1 st periodic table of the elements. Arranged elements in order of increasing atomic mass and created columns with elements having similar properties.
- 48. Mendeleev’s Table Drawbacks Tellurium and Iodine Potassium and Argon Cobalt and Nickel
- 49. Henry Moseley (1887 – 1915) Arranged elements in order of increasing atomic number thus reversing the order of the elements and correcting the drawbacks found in Mendeleev’s table.
- 50. Periodic Law Periodic law states the properties of the elements are periodic functions of their atomic number. In other words, when the elements are listed in order of atomic number, elements with similar properties appear periodically. Therefore, elements in the same column have similar properties. Periodic – to appear at regular intervals
- 51. Modern Periodic Table Period – Row on the periodic table. Periods reflect the energy level of the electrons. .
- 52. A Group or Family is a column on the periodic table. Elements in the same column have similar chemical properties.
- 53. Metals are elements located to the left of the jagged stairs except hydrogen.
- 54. Properties of Metals Metals are solids except mercury, which is a liquid . Metals have luster , are malleable , ductile , and have high tensile strength . Metals are good conductors of heat and electricity.
- 56. Nonmetals Nonmetals are elements located to the right of the jagged stairs plus hydrogen.
- 57. Properties of Nonmetals Nonmetals are solids or gases except bromine, which is a liquid . Nonmetals are dull , and lack other metallic properties. Nonmetals are generally poor conductors of heat and electricity.
- 58. Metalloids Metalloids are elements bordering the stairs except aluminum. They have properties of metals and nonmetals.
- 59. Metalloids are generally semiconductors which means that they conduct to varying degrees making them useful in the computer industry.
- 60. Group A Elements Group A elements all have electrons in the outer s , or s and p orbitals. The group number indicates the number of valence electrons except with helium which has 2. Examples: IIA - Ca (20) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 VIA – S (16) 1s 2 2s 2 2p 6 3s 2 3p 4
- 61. Group 1 (IA) Elements Group 1(IA) elements are the alkali metals with one valence electron. Alkali metals are soft, silver in color, and are too reactive to be found in nature in their free form. Hydrogen is NOT an alkali metal.
- 62. Group 2 (IIA) Group 2(IIA) elements are the alkaline earth metals with 2 valence electrons. Alkaline earth metals are harder, denser, and stronger than alkali metals. They have higher melting points and are less reactive than alkali metals but are also too reactive to be found in their free state in nature.
- 63. Group 18 (VIIA) Group 18 (VIIA) elements are the noble gases with 8 valence electrons, except helium which has 2. Noble gases are inert (nonreactive) in nature. They do not form ions.
- 64. Group 18 (VIIA) Group 18 (VIIA) elements are the noble gases with 8 valence electrons, except helium which has 2. Noble gases are inert (nonreactive) in nature. They do not form ions.
- 65. Group B Elements Group B elements or transition elements (d block) have electrons in their outer d orbitals. The have varying number of valence electrons but frequently have 2 (with notable exceptions). Example: Zn (30) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10
- 66. Transition elements form very colorful ions in solution.
- 69. Stability of Electron Configurations Octet Rule – Atoms having all their outer s and p orbitals filled are more stable (less reactive) than partially filled orbitals. Therefore, atoms will gain or lose electrons in order to achieve a stable configuration. Stable configurations resemble those of noble gases which have 8 valence electrons.
- 70. Example 1 Li has 3 electrons and it has an electron configuration of 1s 2 2s 1 . Lithium will lose its 2s 1 valence electron to leave a configuration of 1s 2 , the same configuration has helium. By losing this electron, lithium will then have one more proton than electron so the lithium atom will have a + 1 charge.
- 71. Example 2 Oxygen (8) has an electron configuration of 1s 2 2s 2 2p 4 . The oxygen atom will gain two valence electrons to obtain the more stable configuration of 1s 2 2s 2 2p 6 . This is the same configuration as the noble gas, neon. The oxygen atom will have 2 more electrons than protons and will carry a -2 charge.
- 72. Ions An ion is an atom that has gained or lost electrons . Cations are atoms that have lost electrons and therefore have a positive charge. Metals lose electrons to form positive ions, cations. Metals lose all their valence electrons. Therefore, their ions are positive by the number they lose.
- 73. Anions An anion is an atom that has gained electrons and therefore, has a negative charge. Anion named end in –ide. S -2 is called the sulfide ion. Nonmetals gain electrons to from negative ion, anions. They gain electrons to have a stable octet (8) of electrons. Therefore, nonmetal ions are negative by the number of electrons they gain.
- 76. Ion Formulas Ion formulas consist of the element’s symbol followed by its charge or oxidation state. Rubidium - Rb +1 Iron – Fe +2 Aluminum – Al +3 Lead – Pb +4 Sulfur – S -2 Iodine – I -1 Nitrogen – N -3 Hydrogen – H +1 or H -1
- 77. Exceptions to Predicted Electron Configurations According to the octet rule, filled and half-filled sublevels are more stable (less reactive). Therefore, in some cases, actual configuration varies from predicted configurations
- 78. Exceptions of the Octet Rule Predicted Configuration Chromium Cr 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 4 Actual Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5
- 79. Exceptions to the Octet Rule Predicted Configuration Copper Cu 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 Actual Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 10
- 80. Periodic Properties As you have seen, elements in the same row are similar in their outer electron configurations. This results in these elements having relatively the same physical properties such as density, melting point, and boiling. These physical properties are then said to be periodic properties.
- 81. Periodic Trends A periodic trend is a general tendency that occurs across periods or within groups in the periodic table. Exceptions are always present in trends.
- 83. Atomic Radii Atomic radii is the radius of an atom. Down a Group – radius increases. Reasons – * Addition of energy levels * Shielding of outer electrons from the nucleus by inner electrons in larger atoms. * Electron – electron repulsion in outer energy levels
- 84. Across a Period Across a Period – Radius Decreases Reasons – 1. no addition of energy levels 2. increased nuclear charge causes electrons to be pulled closer
- 86. Atomic Radius Vs. Ion Radius The radius of the ion formed from an atom will be smaller or larger than the radius of the original atom.
- 87. If the original atom is a metal then the atom will lose electrons to form a positive ion. This results in the in the ion having a smaller radius than the original atom.
- 88. If the original atom is a nonmetal, the ion is formed when the atom gains electrons. This will result in the ion having a larger radius than the original atom.
- 89. First Ionization Energy 1 st Ionization Energy is the energy required to remove an electron from an atom. Down a Group – Ionization Energy Decreases Reason – Outer electrons in larger atoms are held more loosely by the nucleus.
- 90. 1 st Ionization Energy Across a Period – Ionization Energy Increases Reasons – 1. Outer electrons in smaller atoms are held more tightly by the nucleus. 2. An octet of electrons is approached.
- 91. Periodic Trend for 1 st Ionization Energy