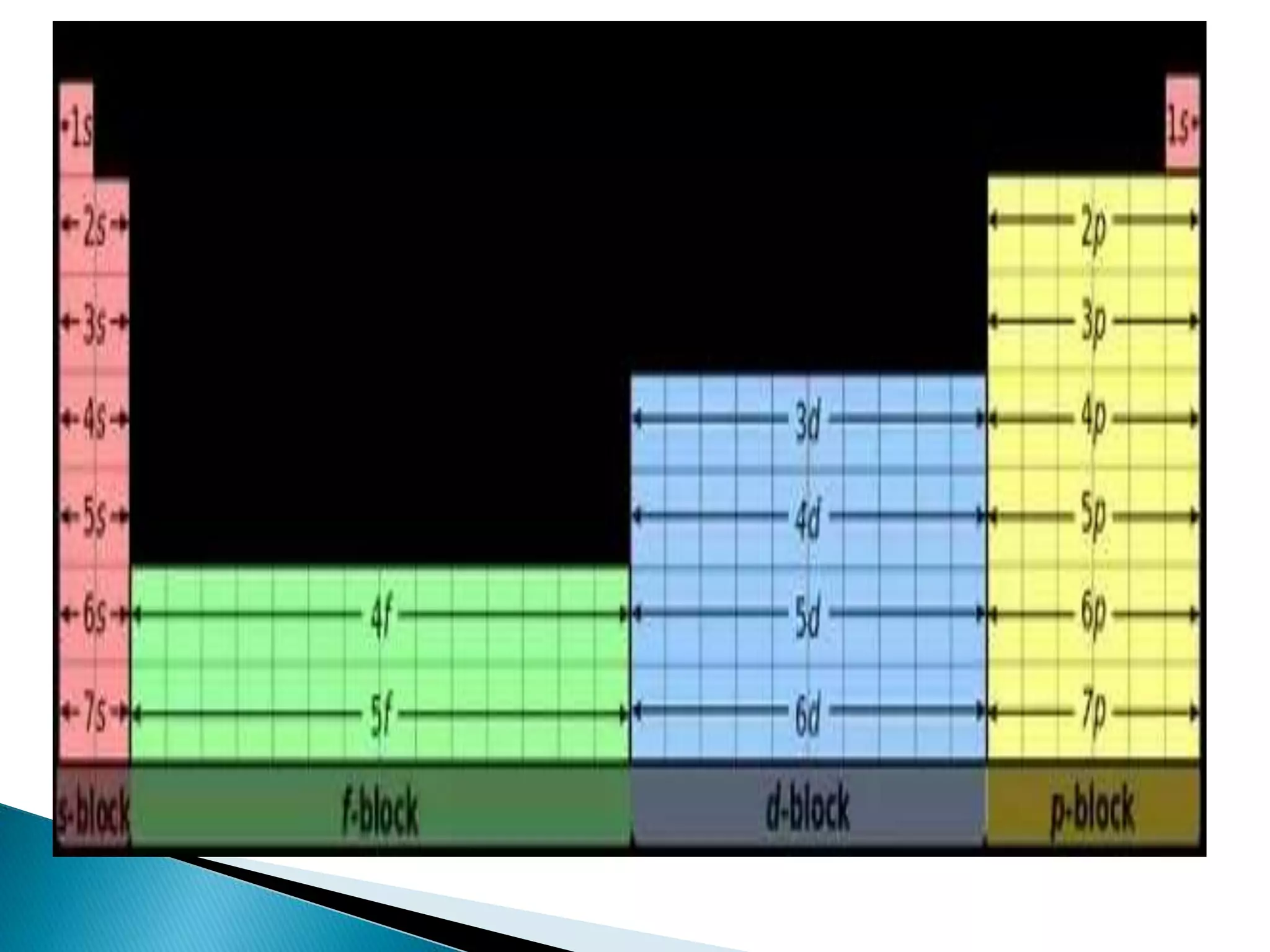
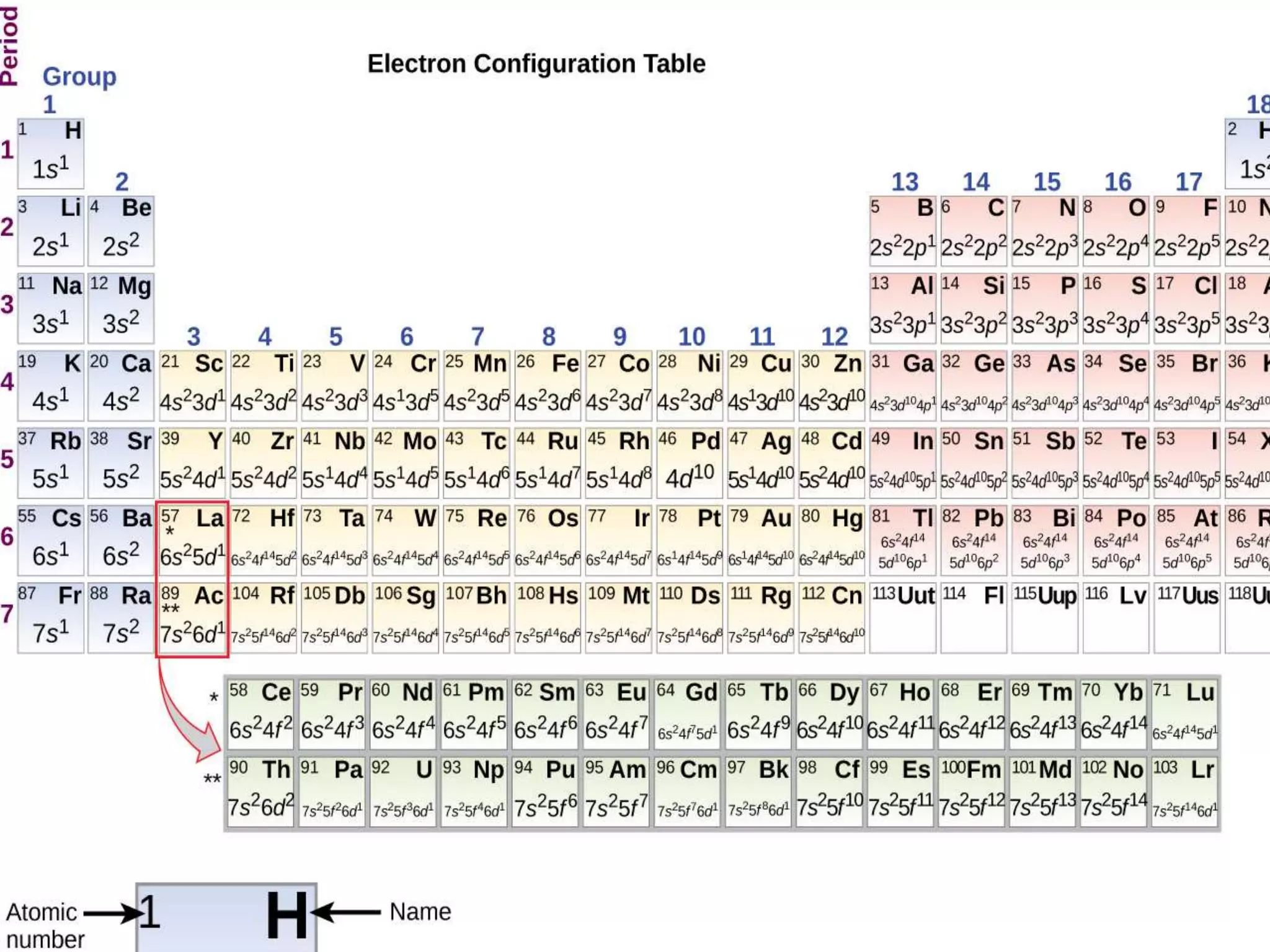
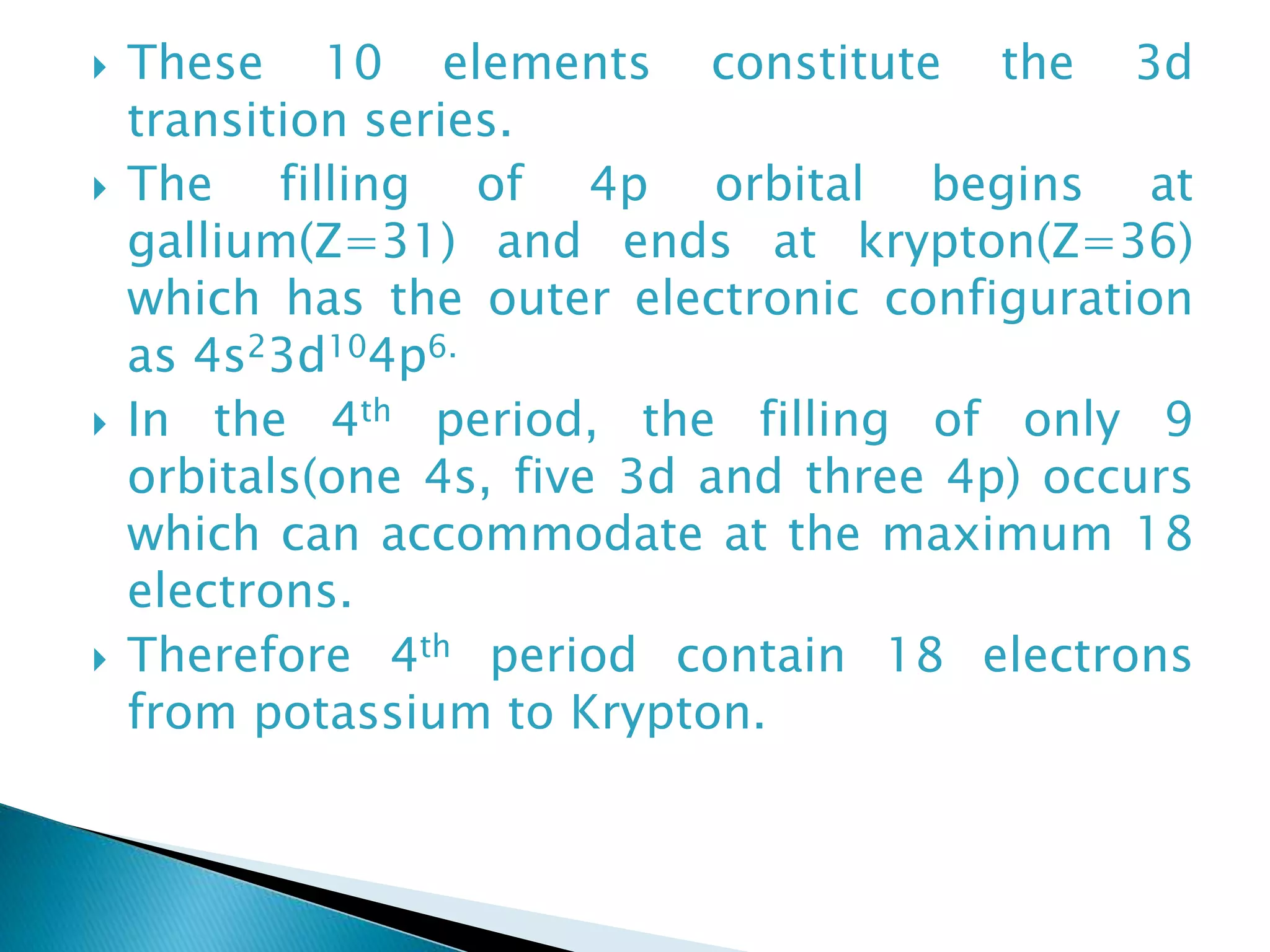
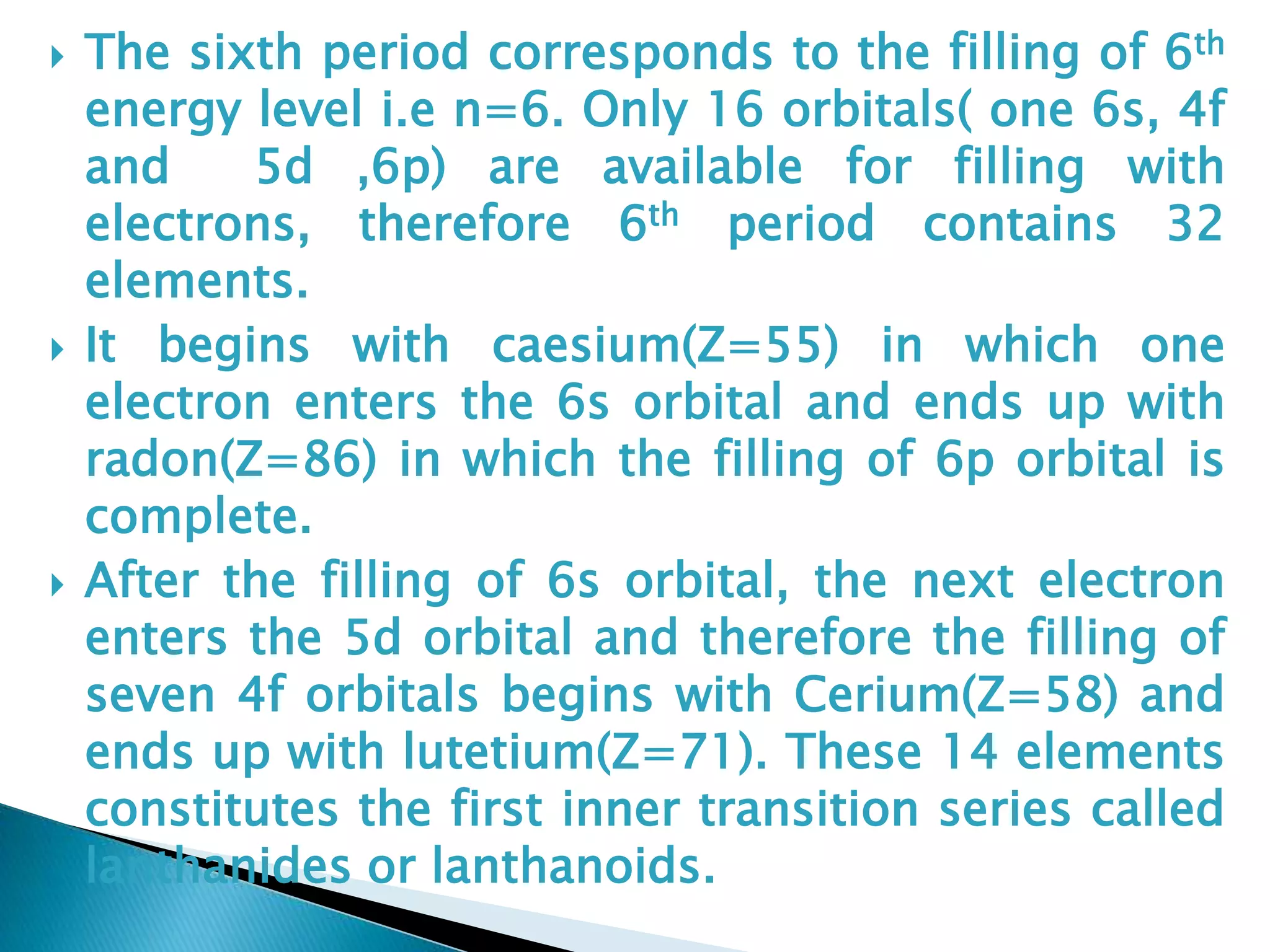
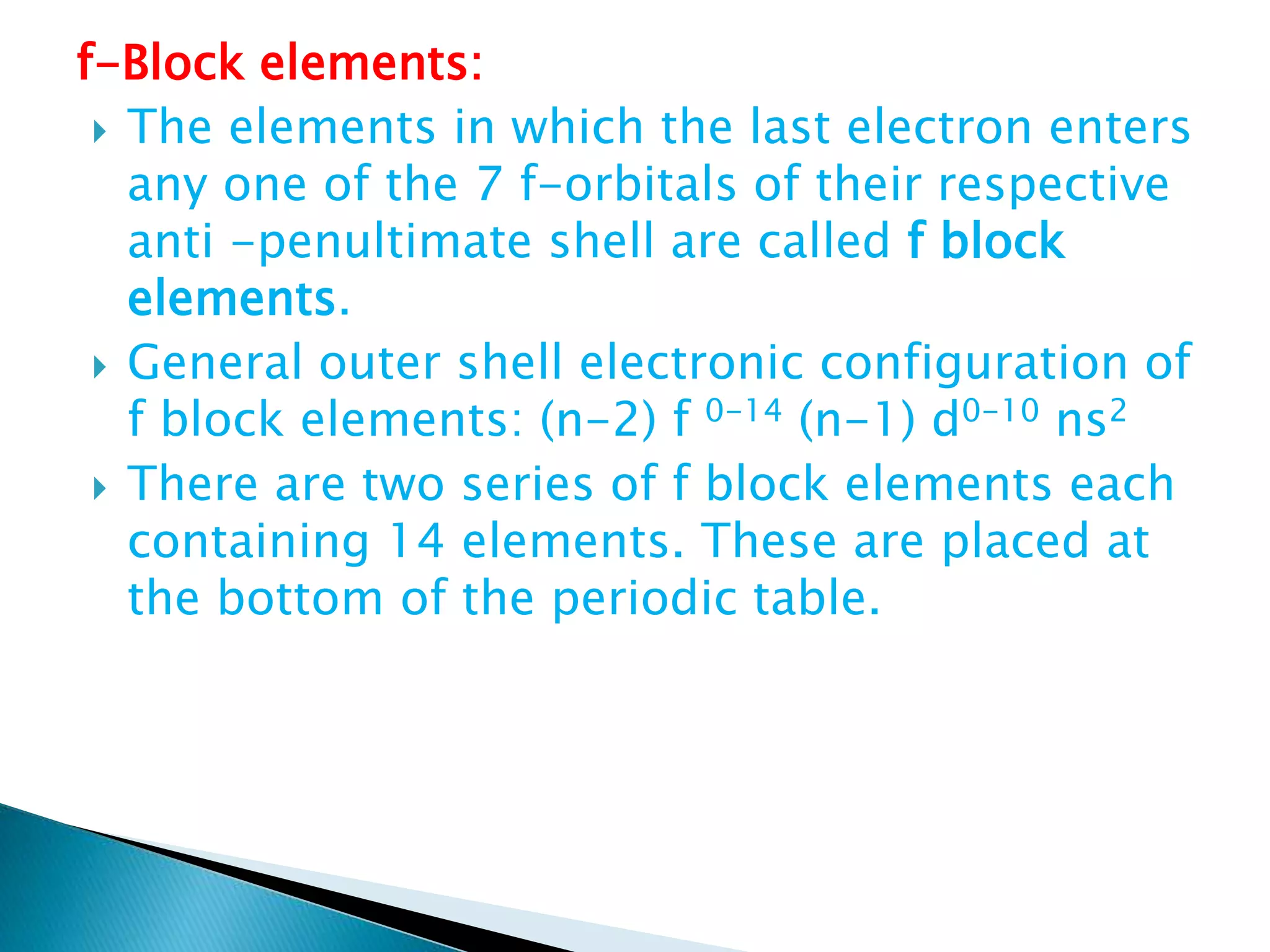
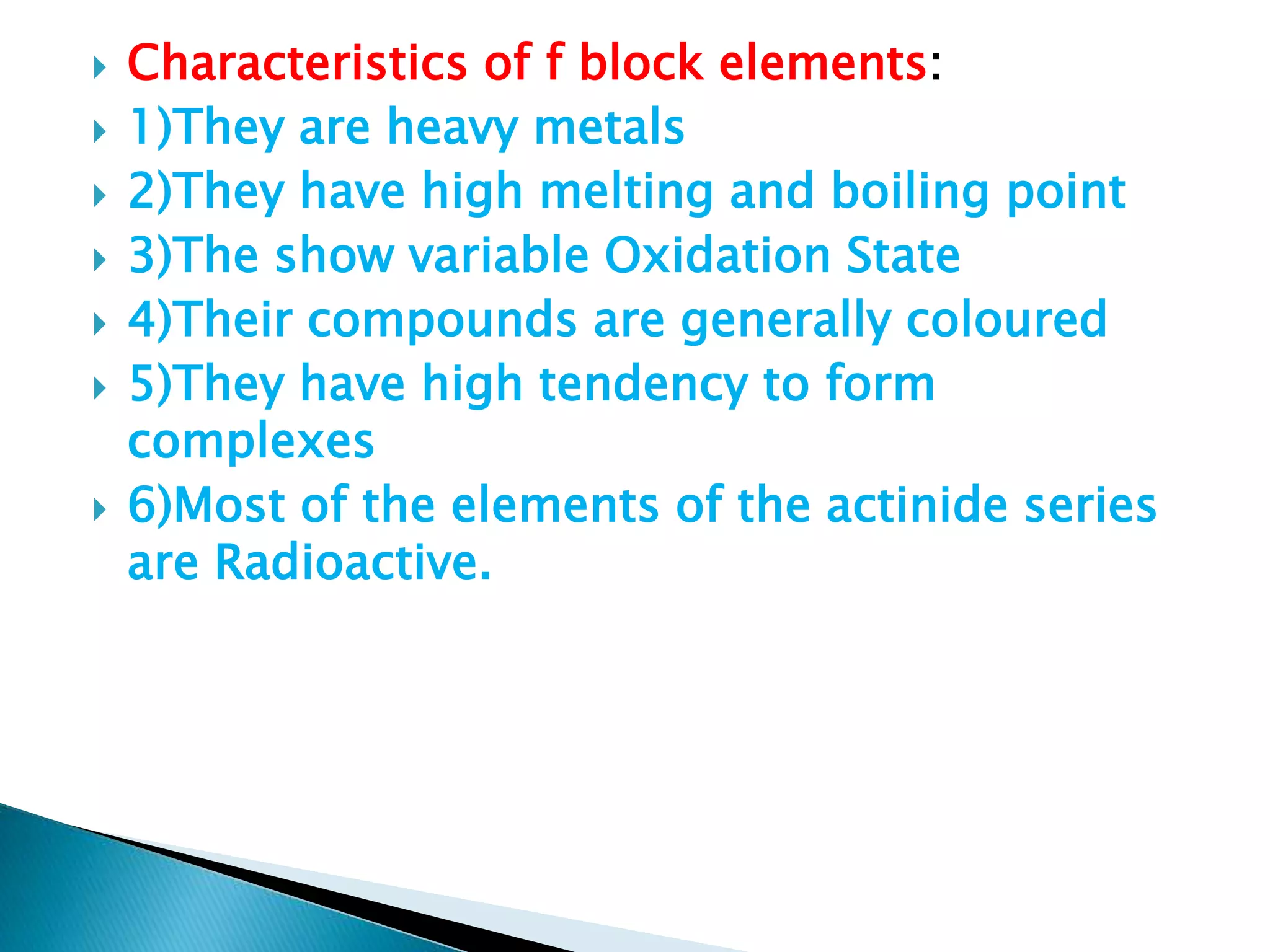
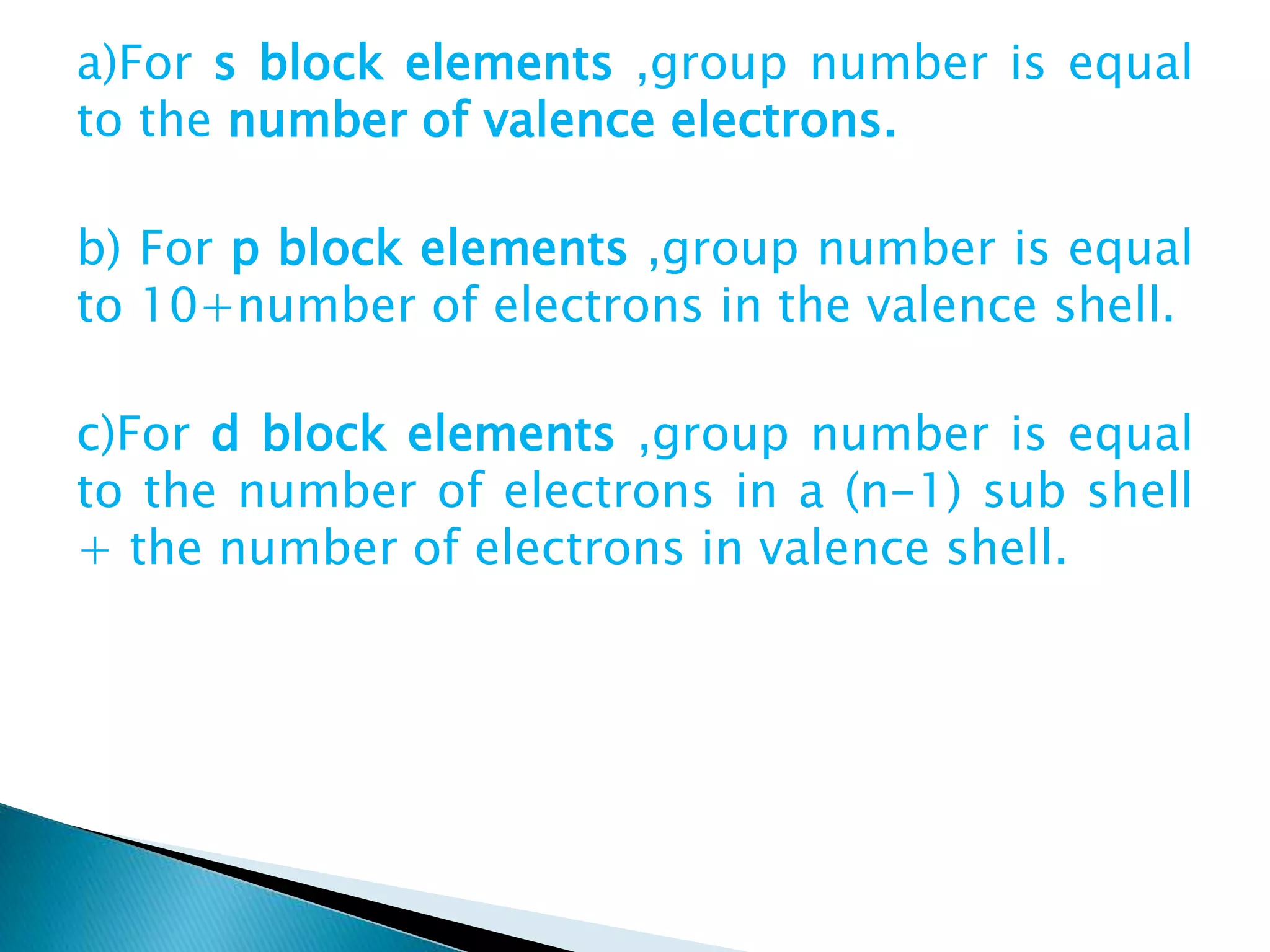
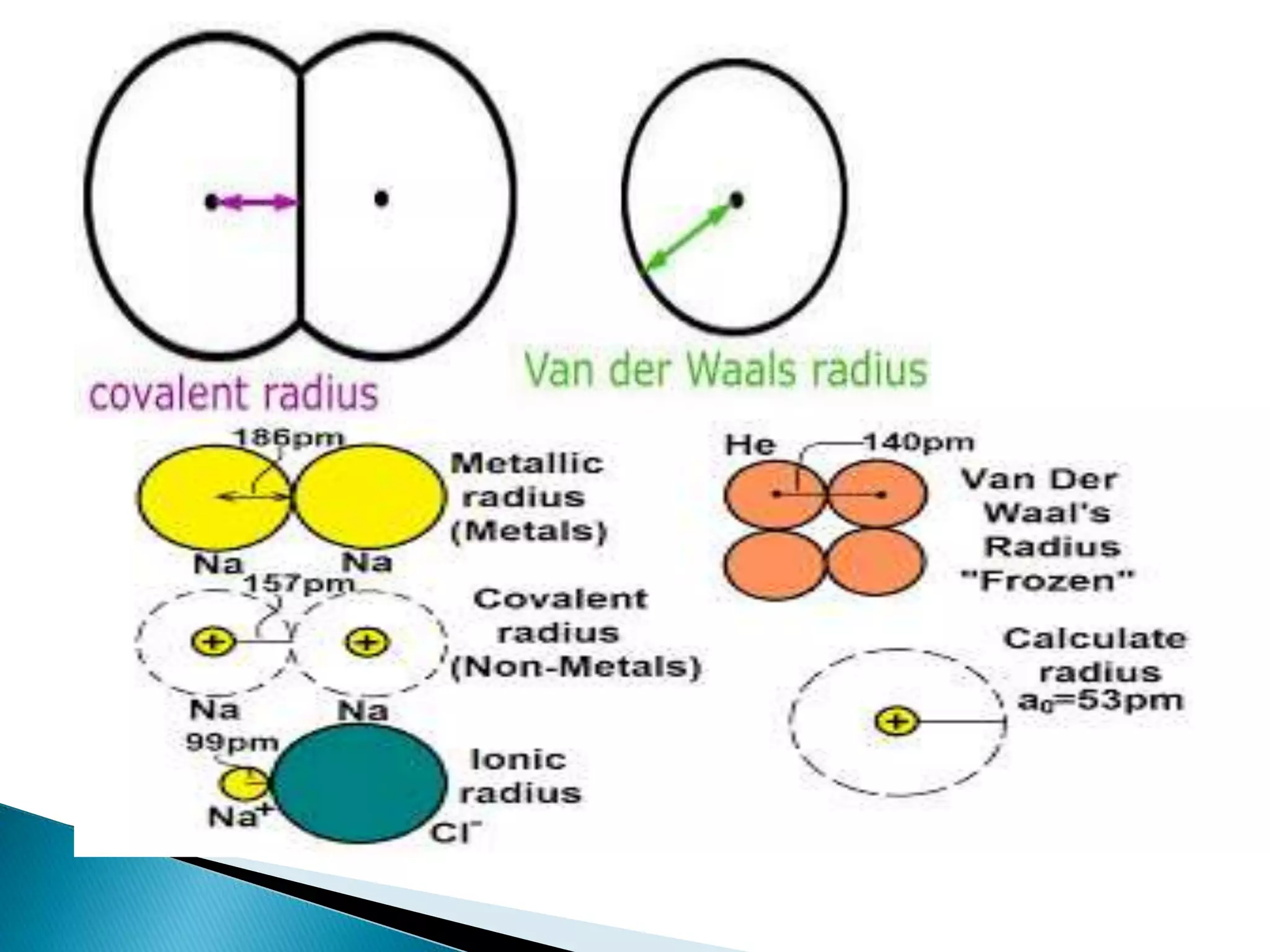
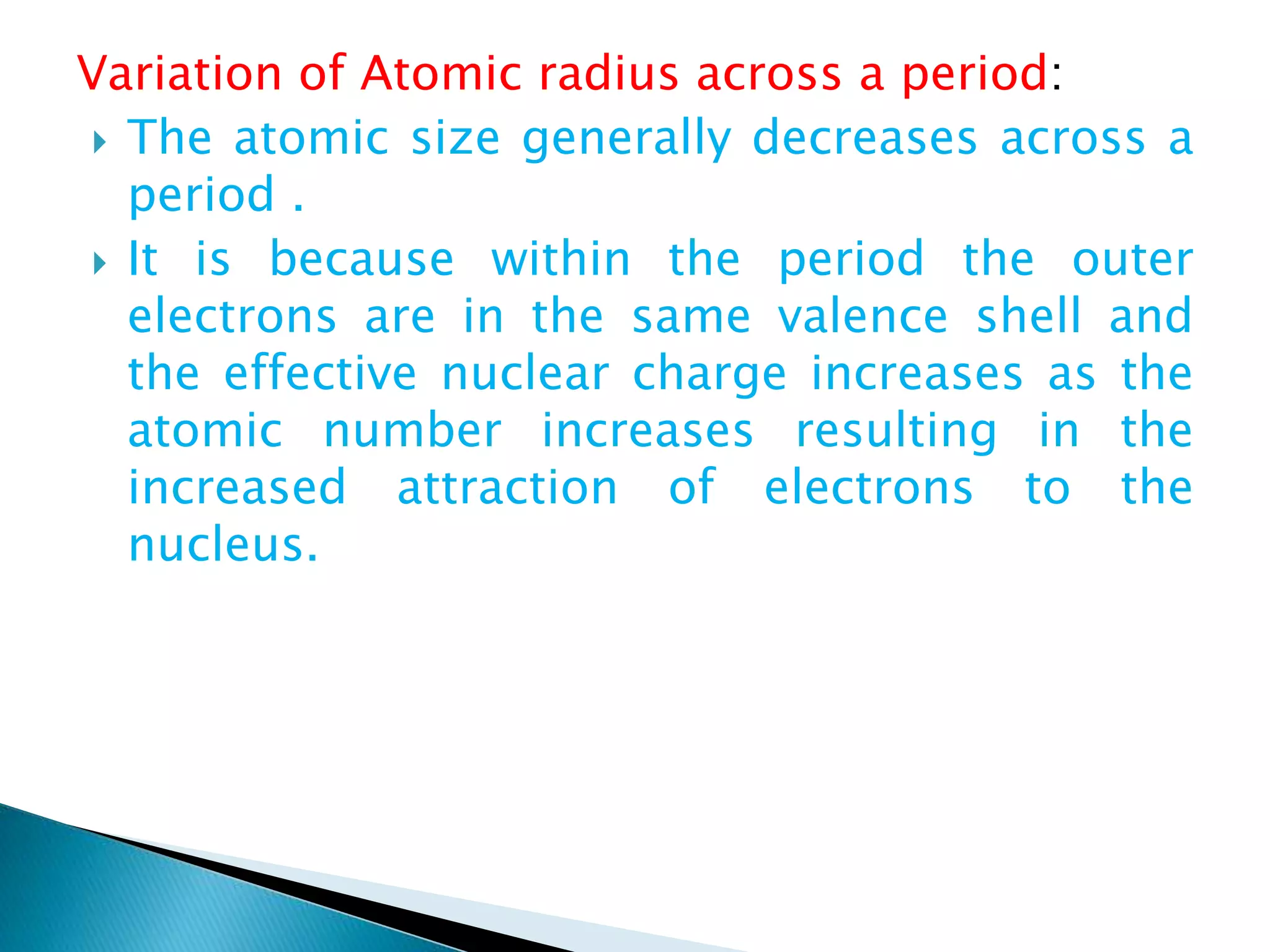
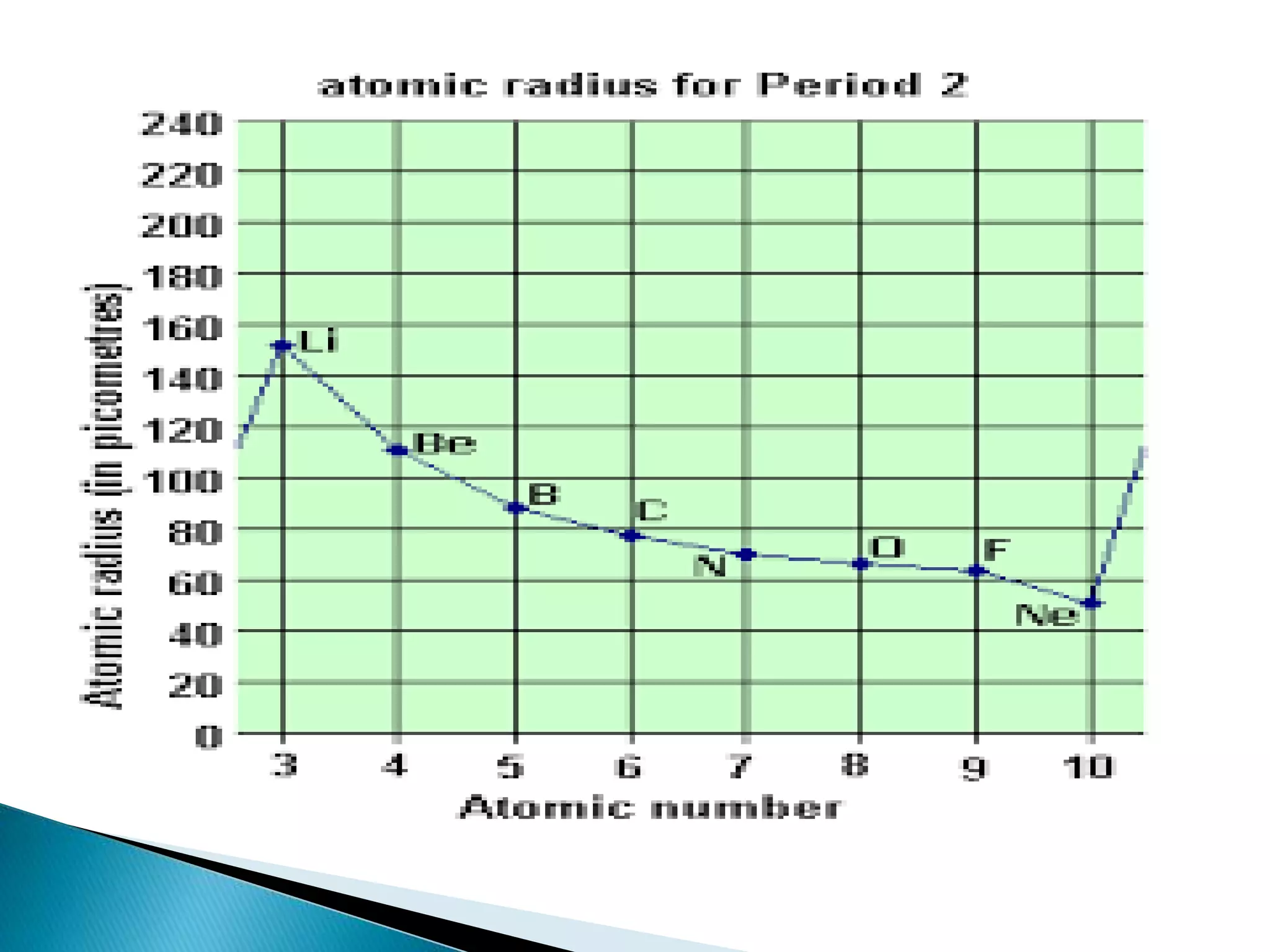
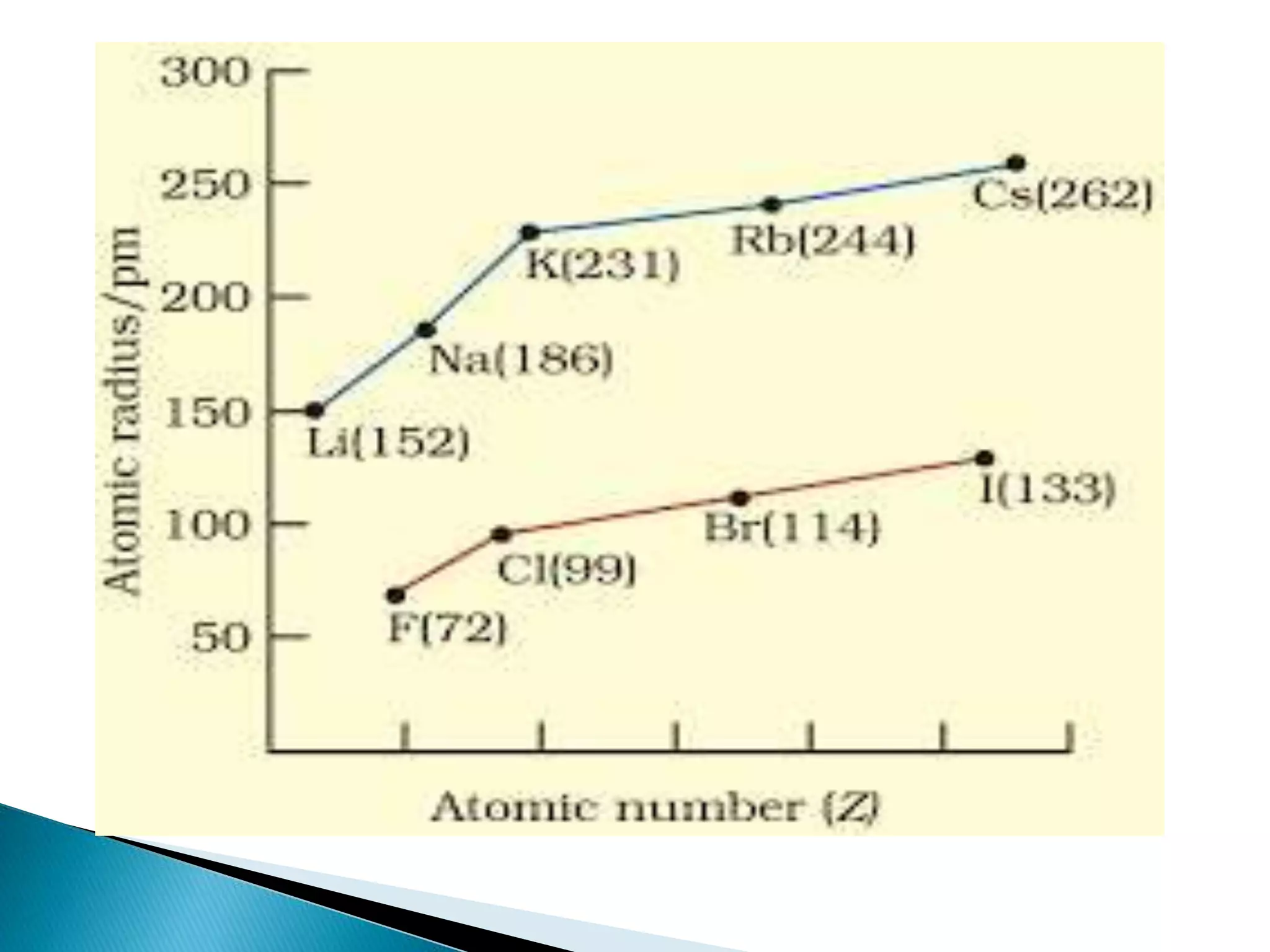
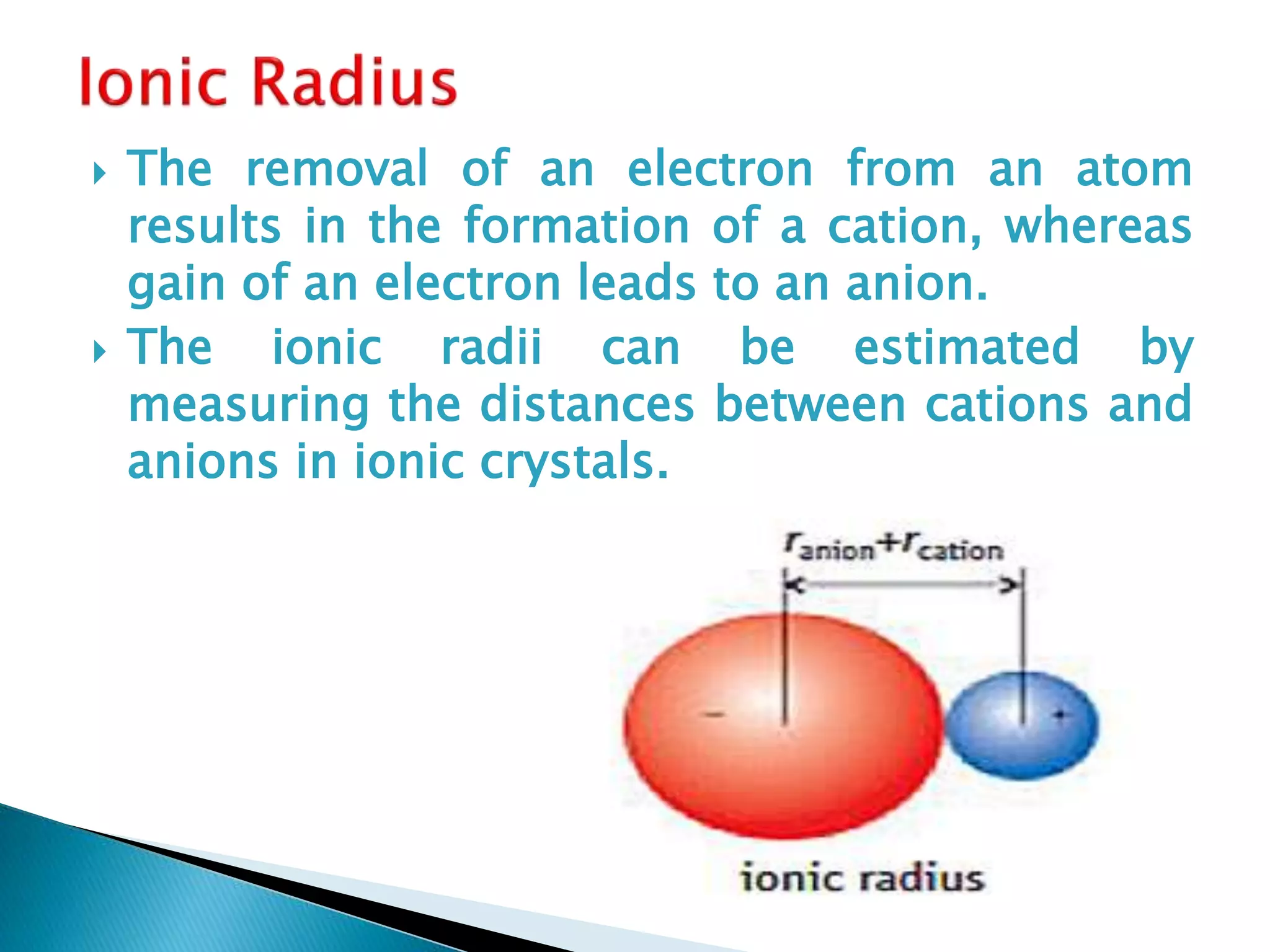
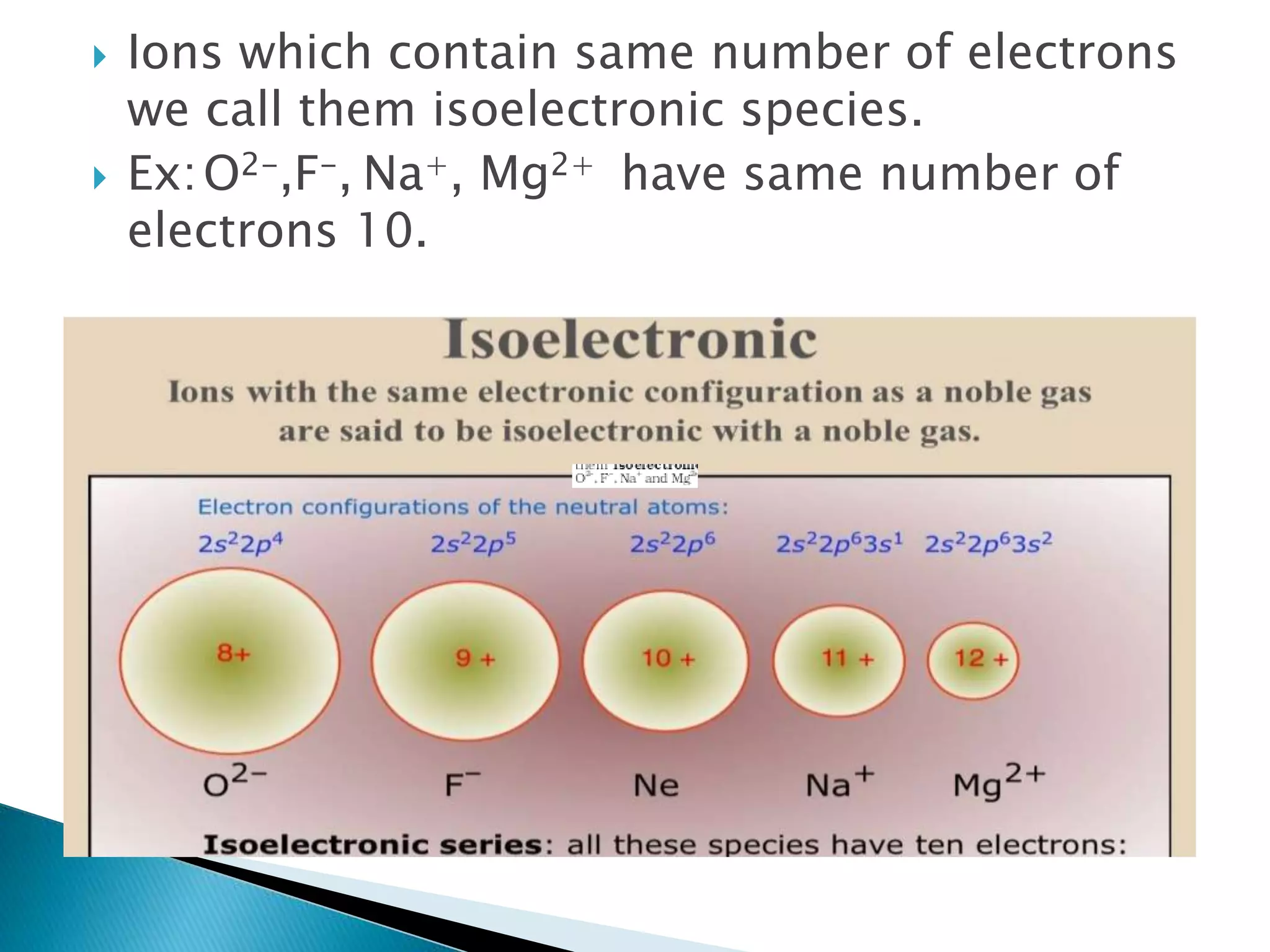
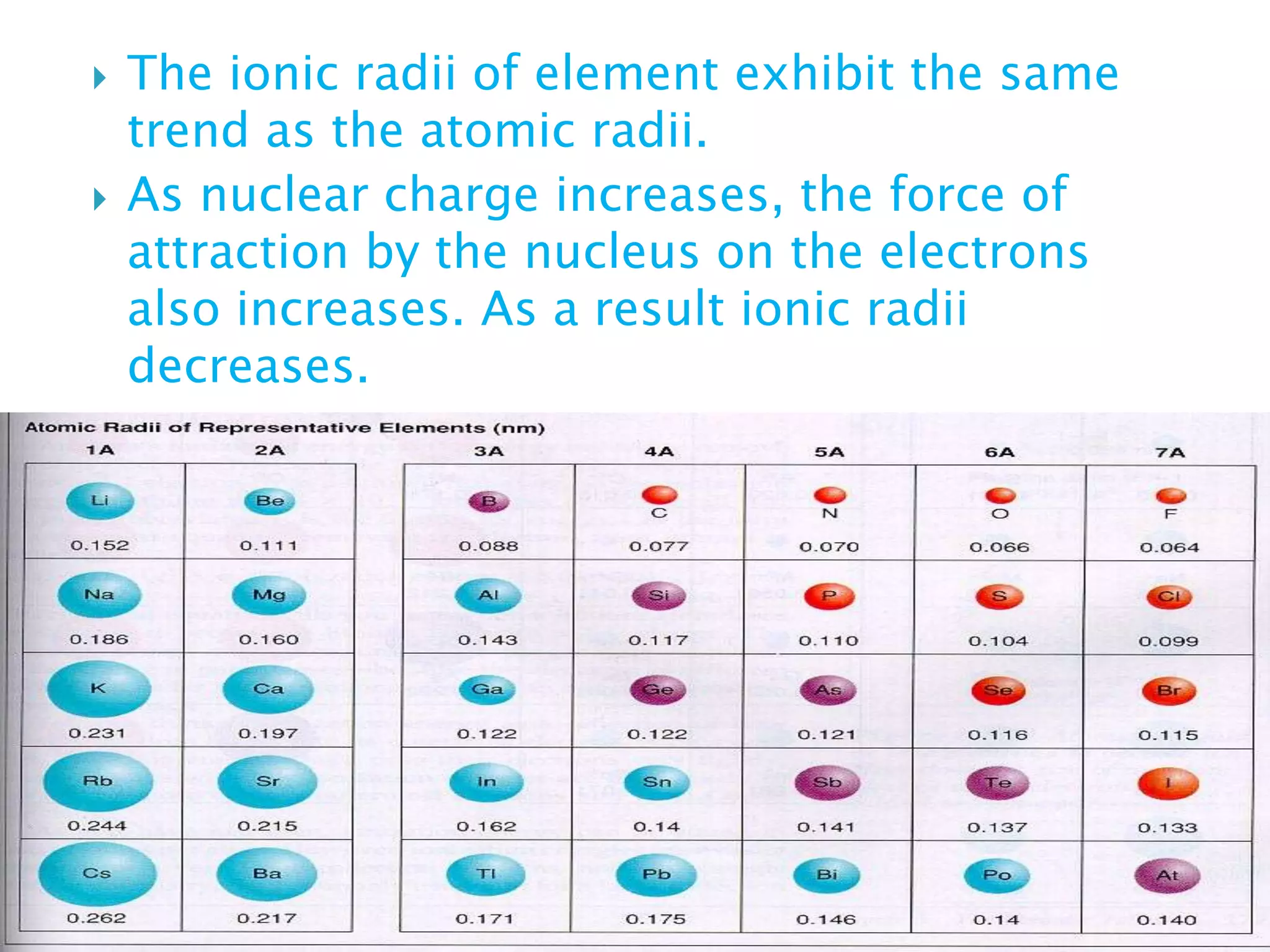
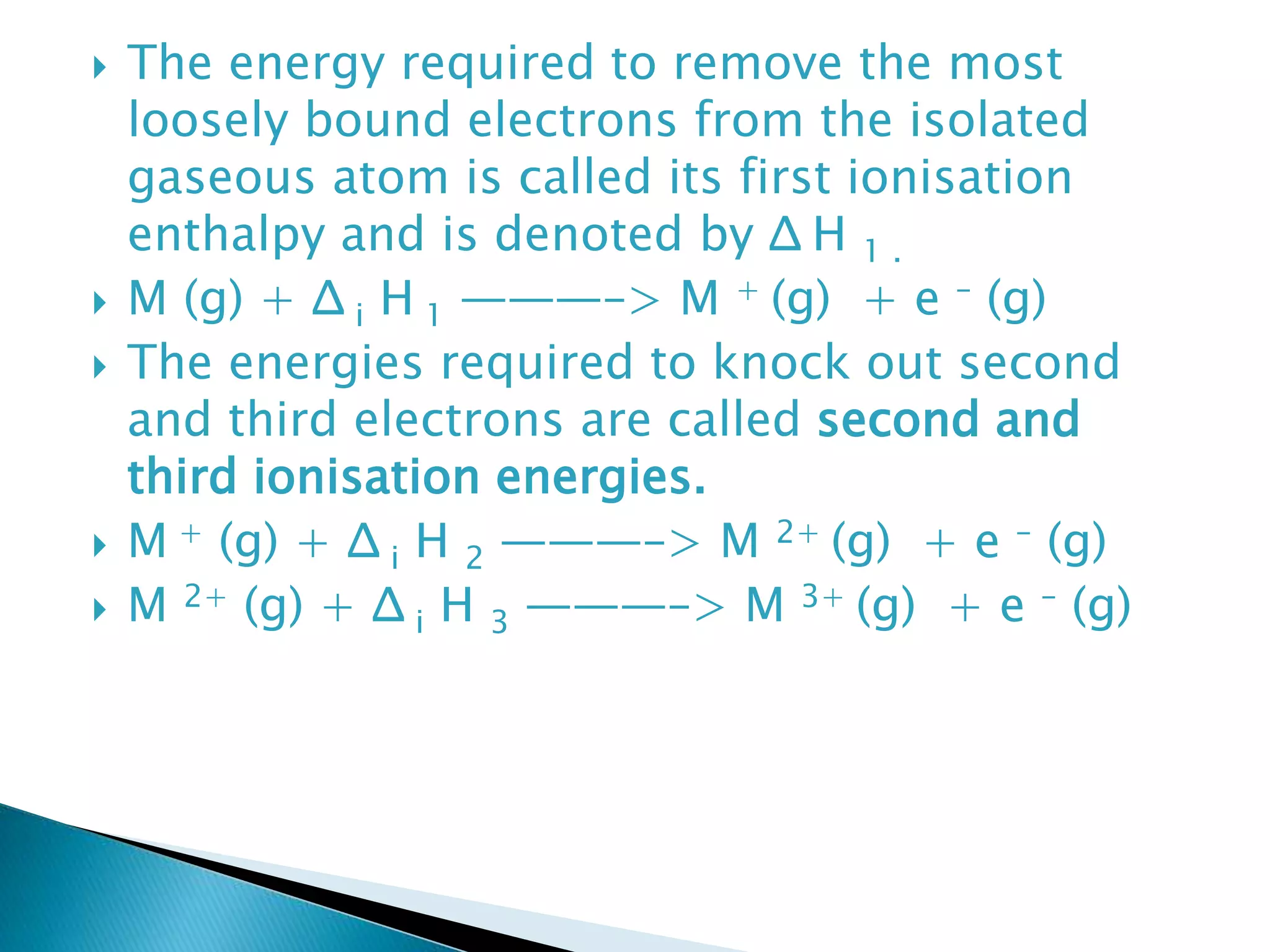
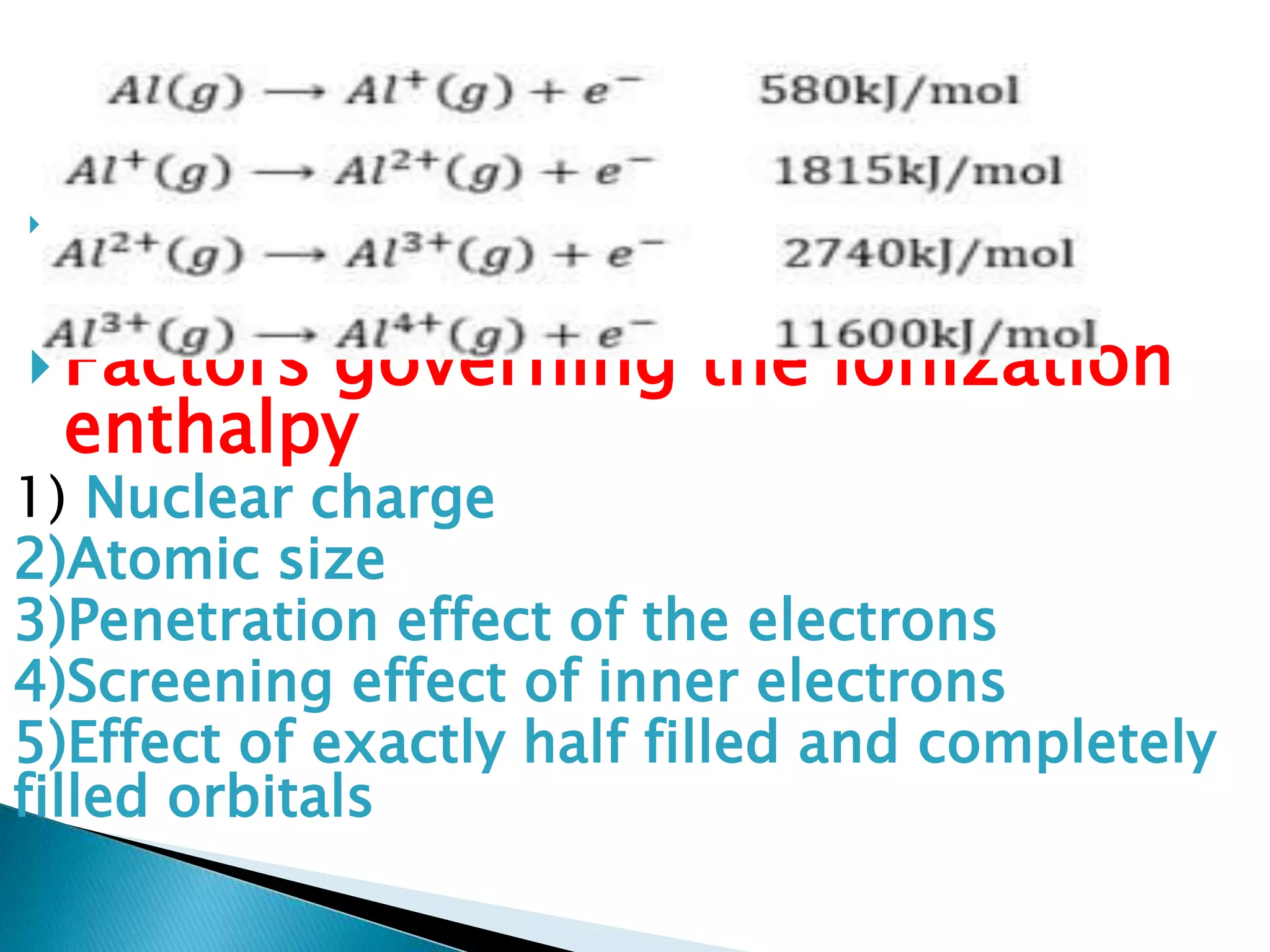
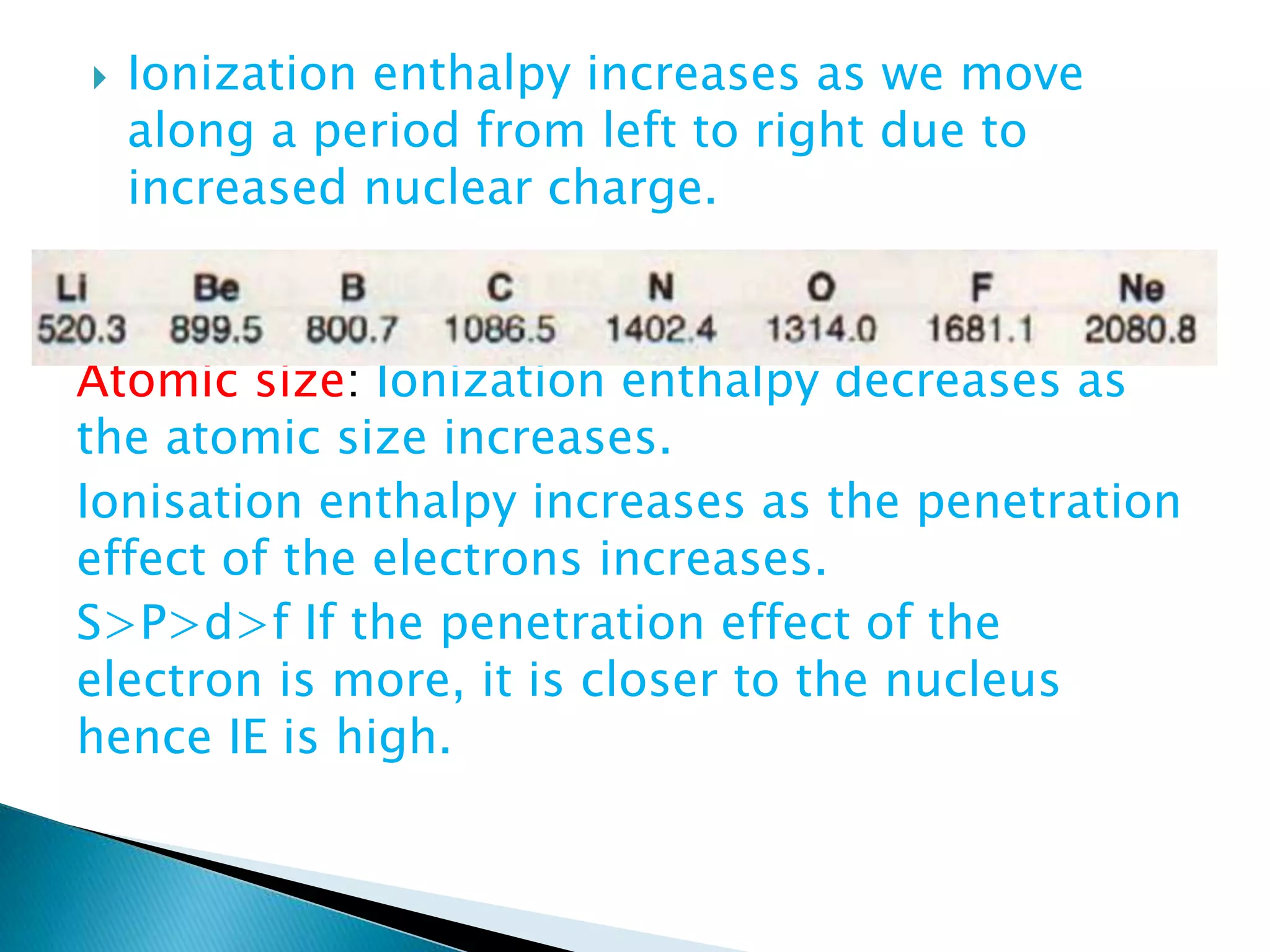
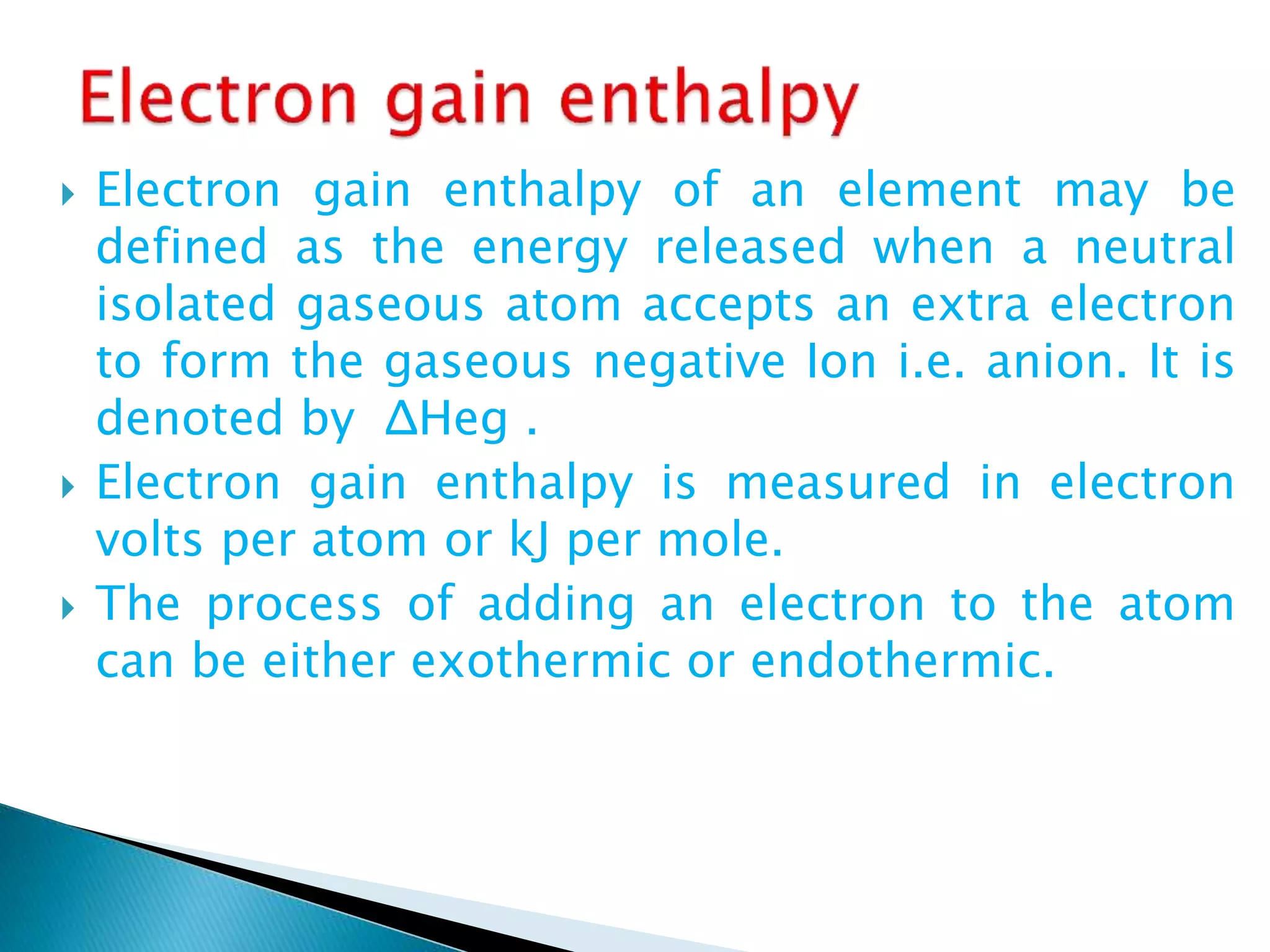
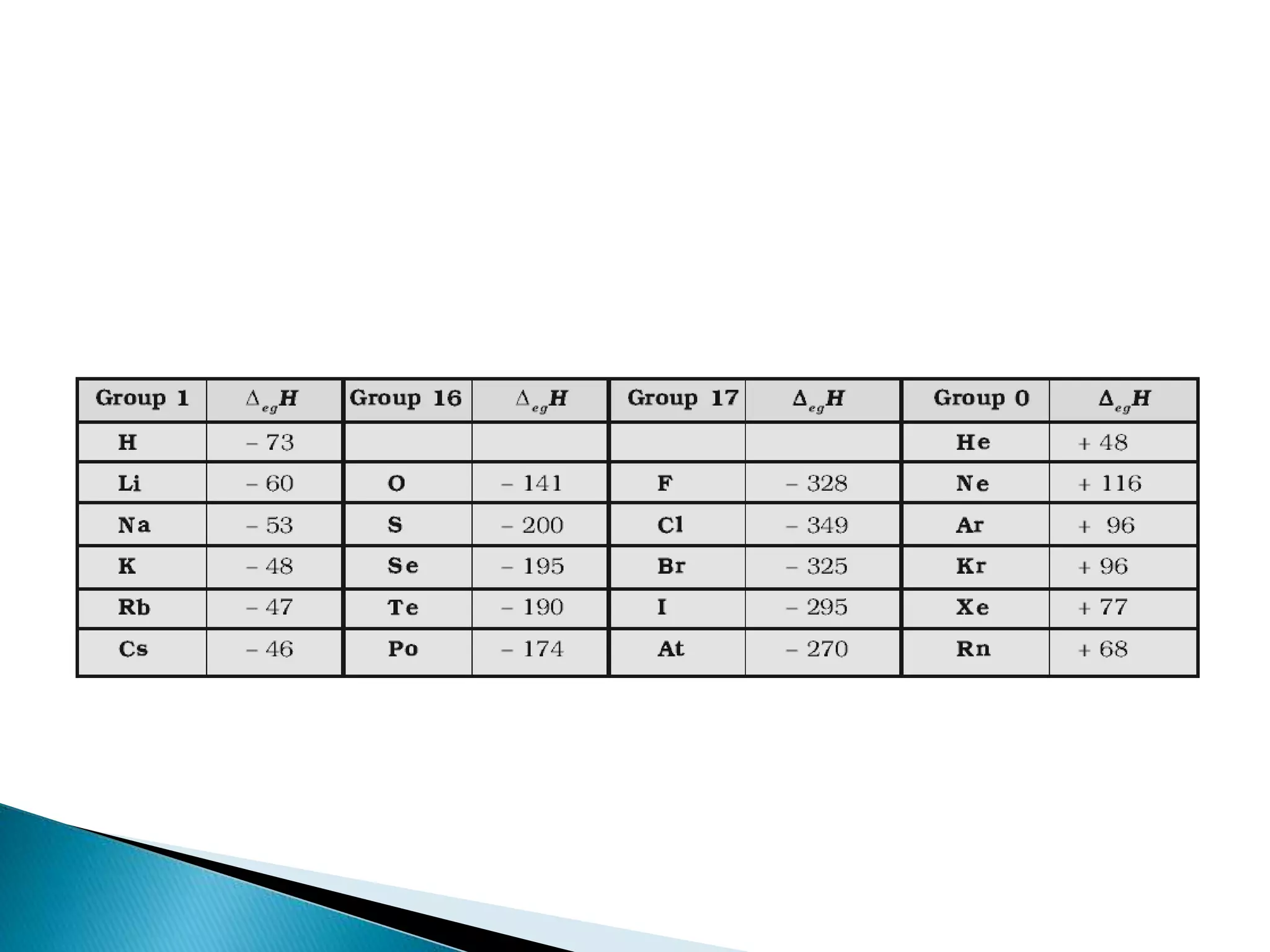
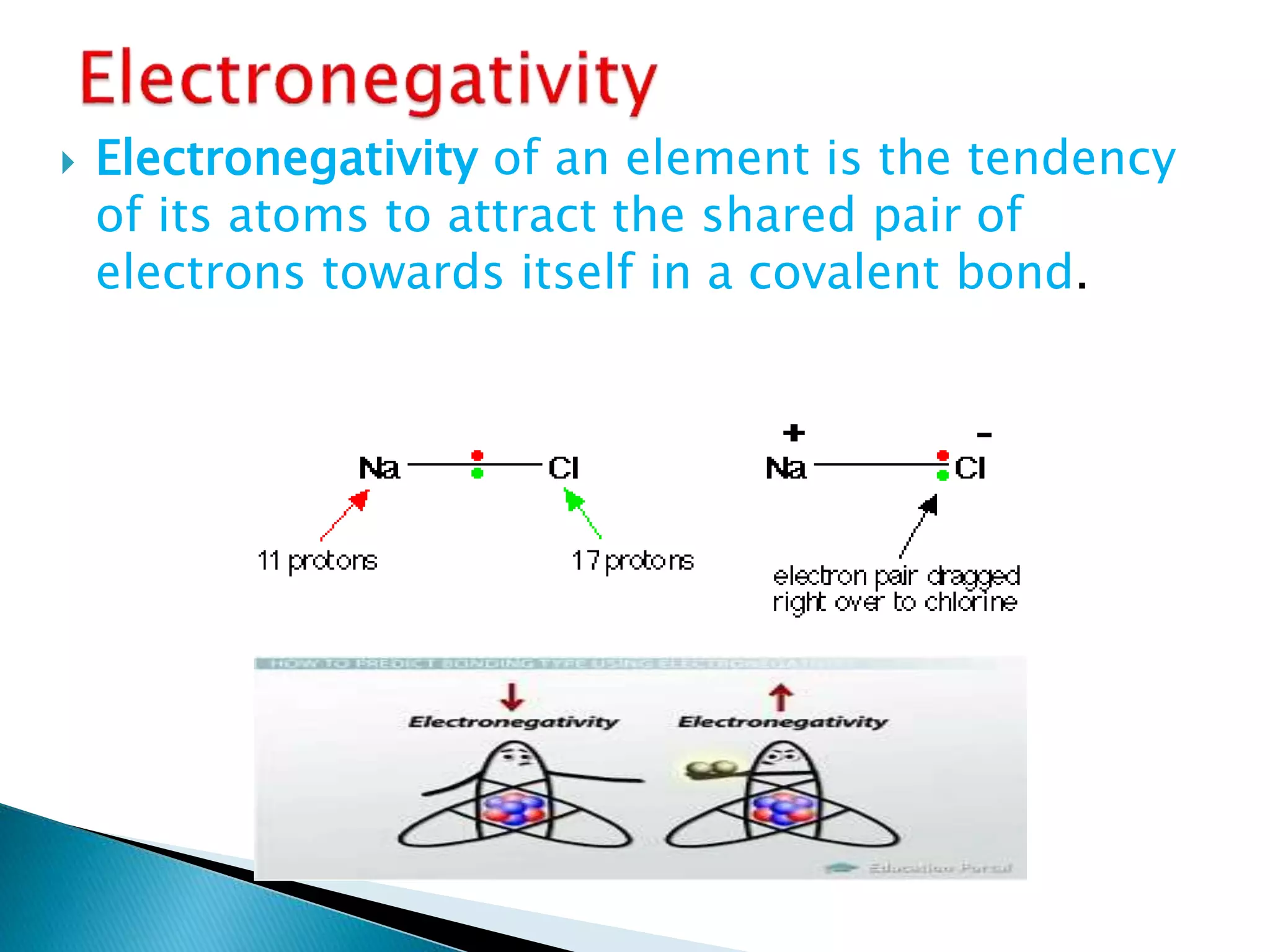
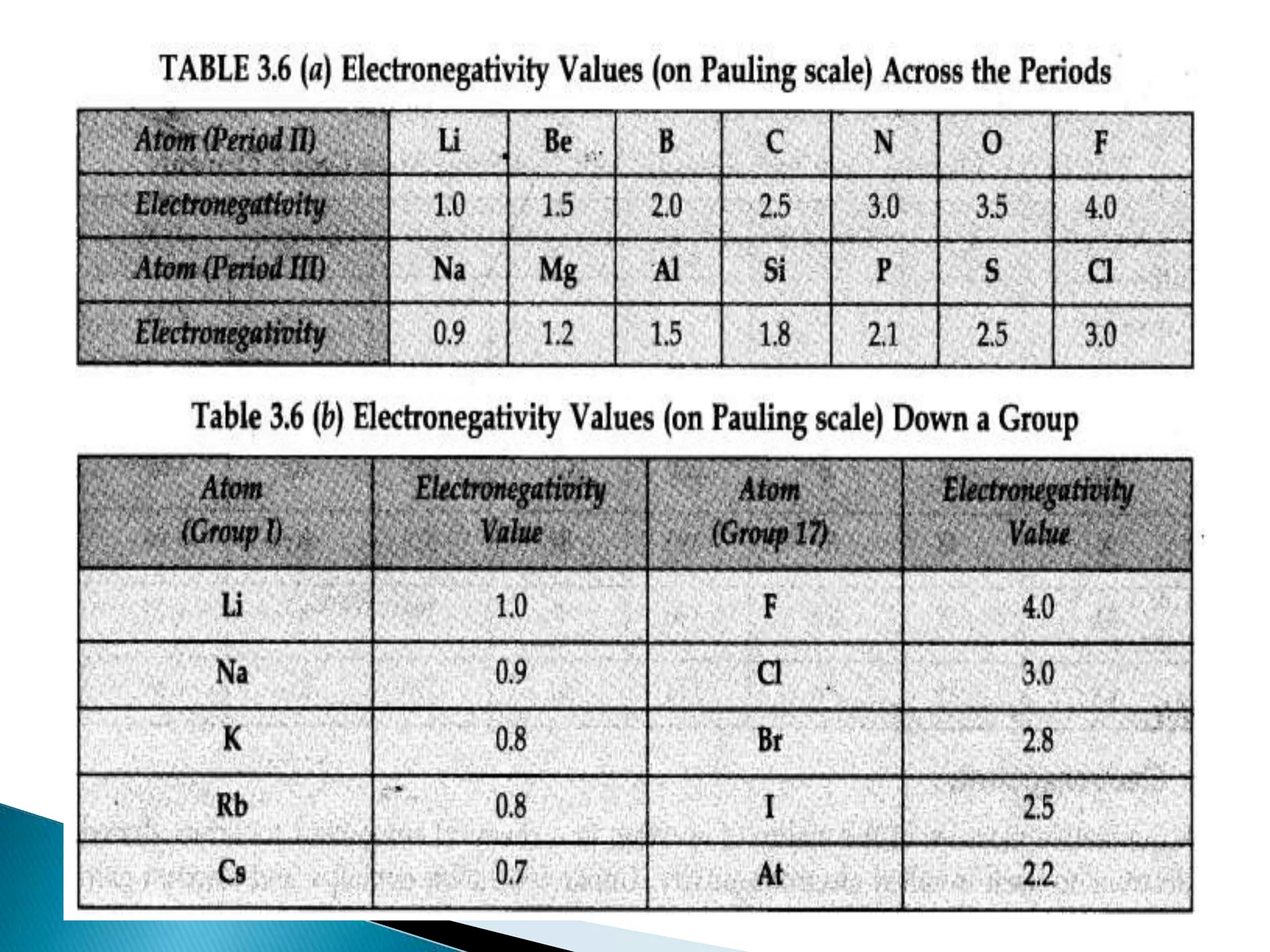
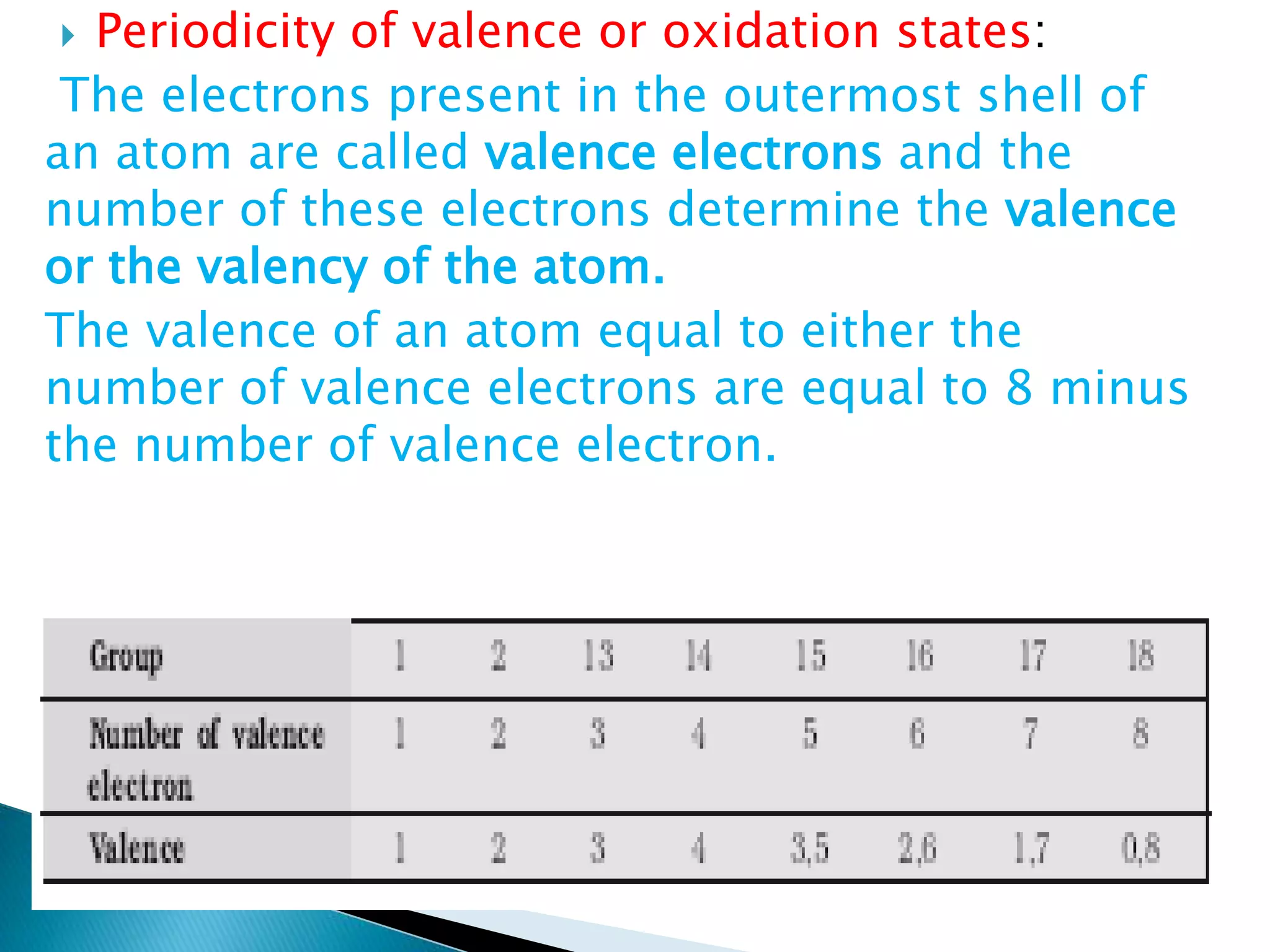
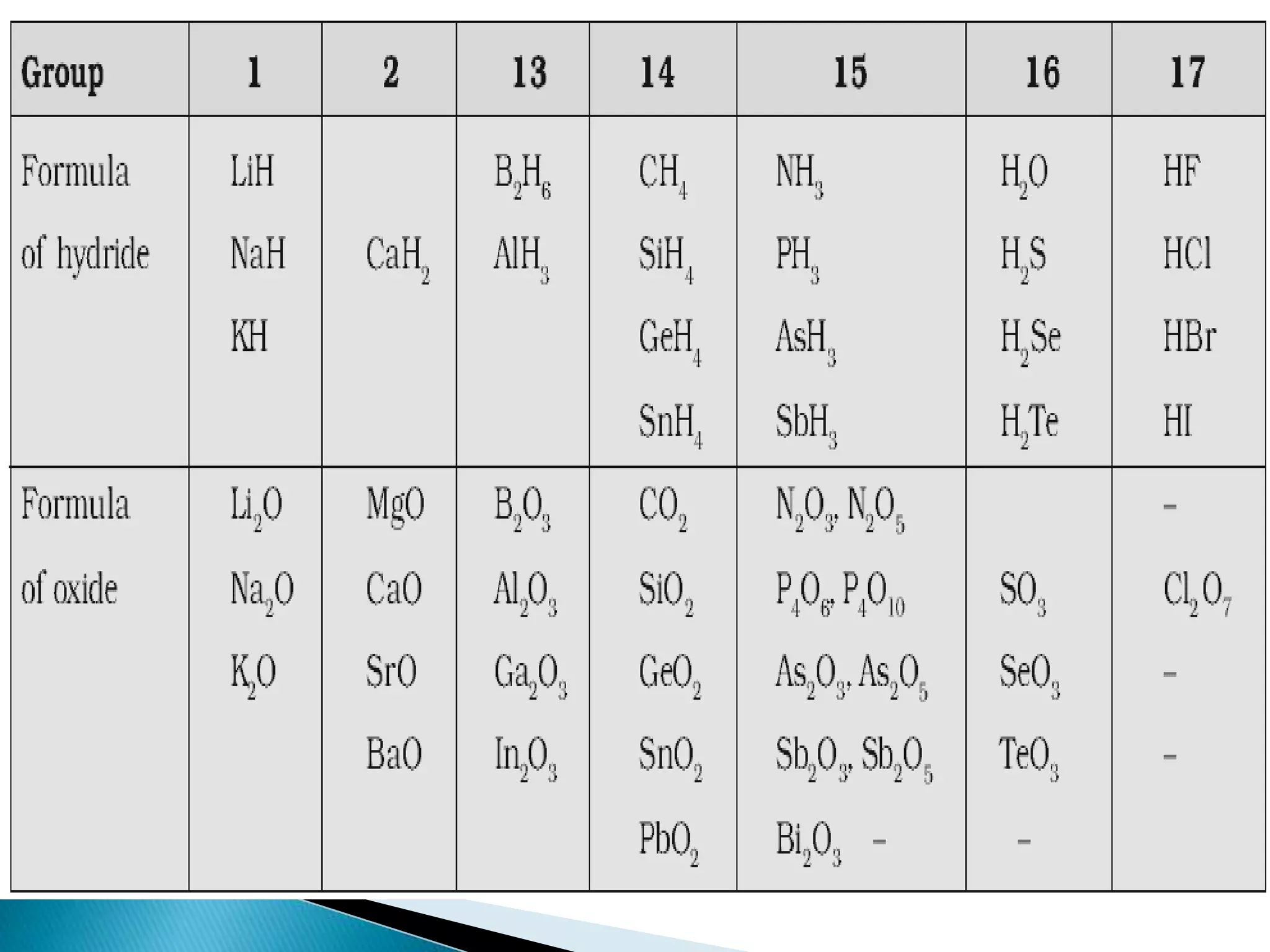
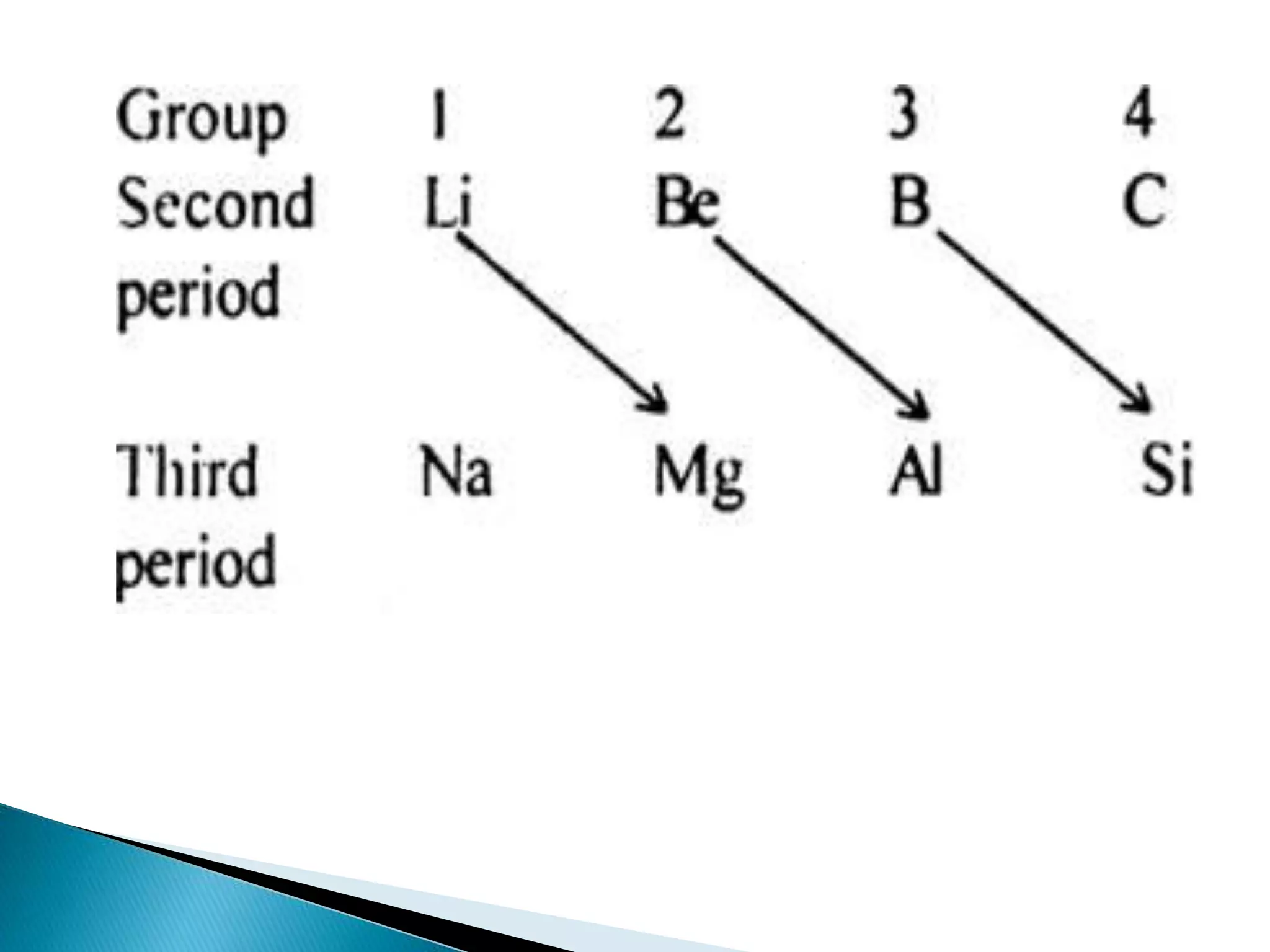
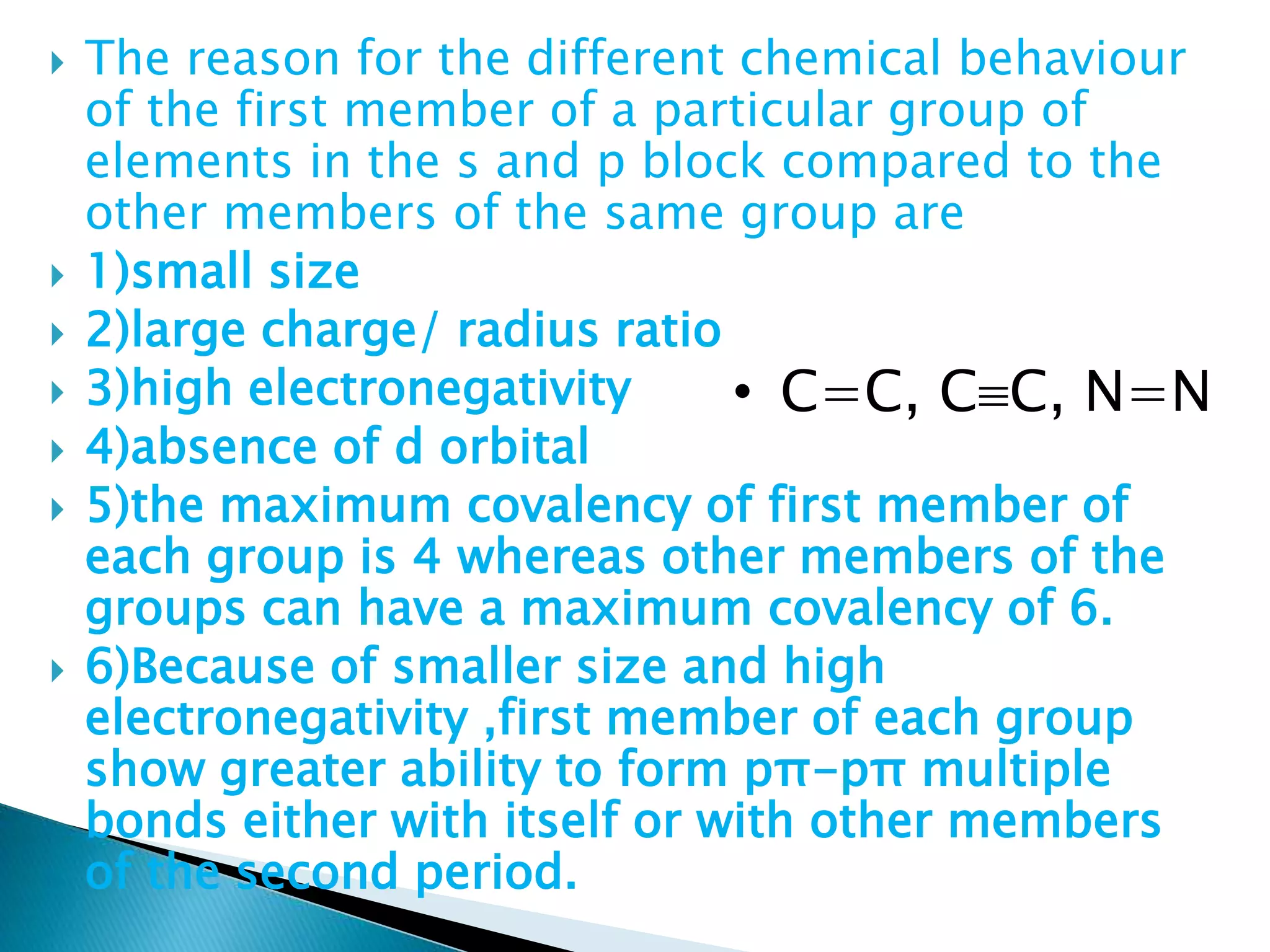
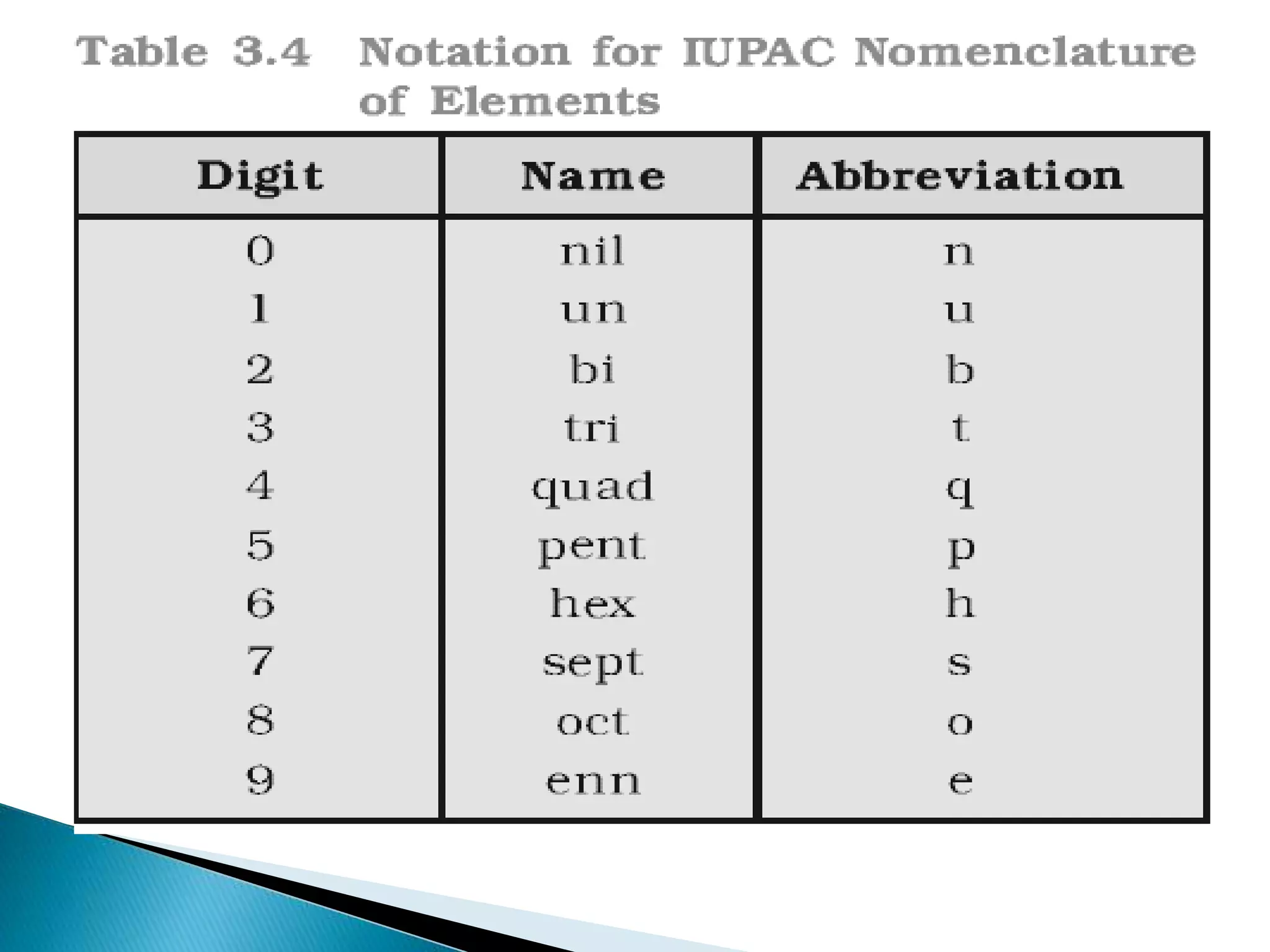
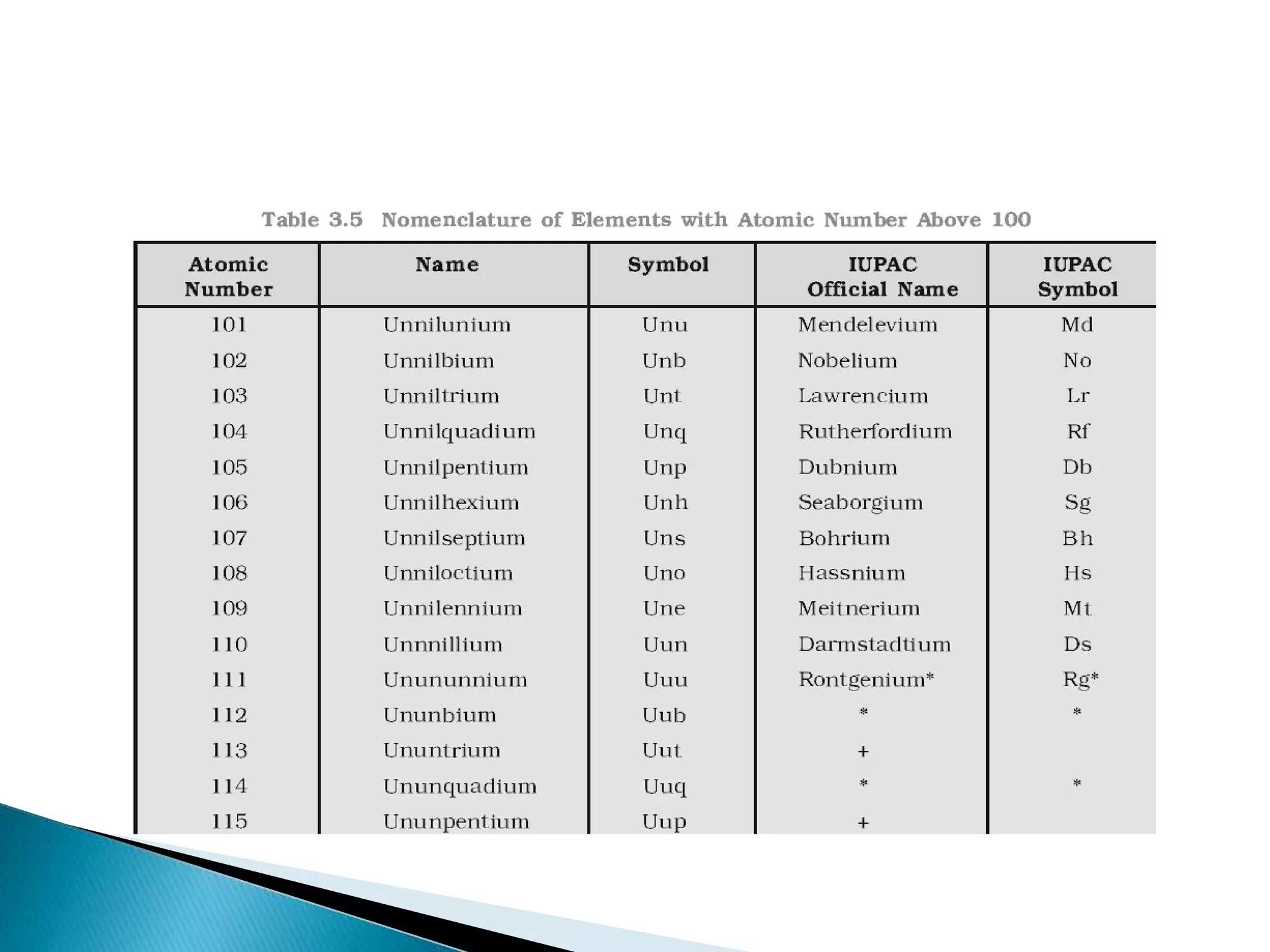
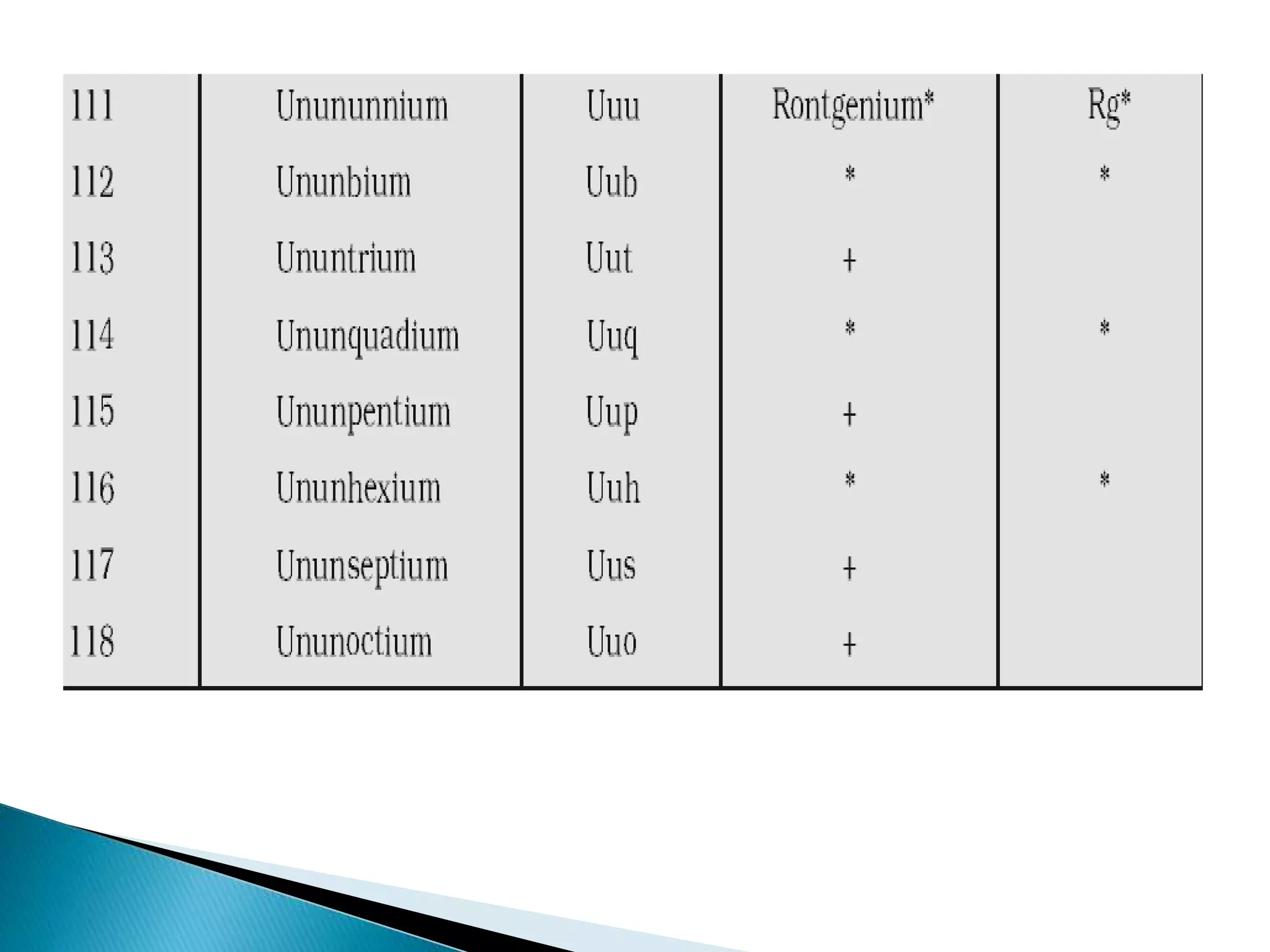
The document provides detailed notes on the modern periodic law, the structure of the periodic table, and trends in properties such as atomic and ionic radii, ionization enthalpy, and electronegativity. It explains how elements are organized in periods and groups based on electron configurations, detailing characteristics of s, p, d, and f block elements. The notes also cover periodic properties and the variation of atomic and ionic radii across periods and groups.