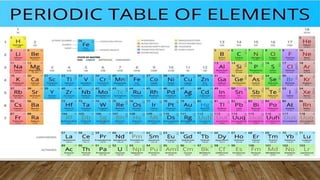
The modern periodic table, introduced by Henry Moseley, arranges 92 known elements by increasing atomic number, emphasizing that elements in the same group have similar physical and chemical properties due to identical valence electron counts. It contains 18 groups and 7 periods, where group number indicates valence electrons and period number reflects electron shell configuration. The placement of elements reveals their chemical reactivity and bonding capabilities.