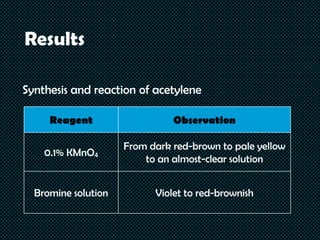
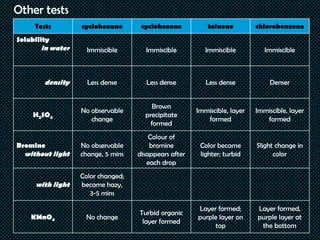
Hydrocarbons are organic compounds made of only carbon and hydrogen. They include fuels, plastics, and other important products. The document describes tests conducted on various hydrocarbons to observe their physical and chemical properties. Acetylene was synthesized from calcium carbide and water, and reacted with bromine solution and potassium permanganate solution. Solubility, density, reactions with sulfuric acid, bromine, and potassium permanganate were tested for cyclohexane, cyclohexene, toluene, and chlorobenzene. The results showed that properties and reactivity differed based on whether the hydrocarbons were saturated, unsaturated, or aromatic compounds.