Embed presentation
Download as PDF, PPTX

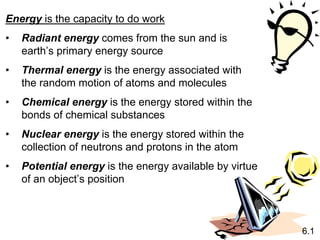
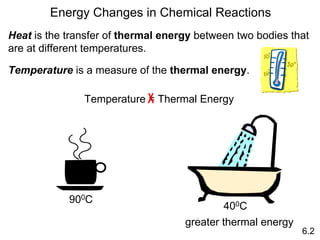


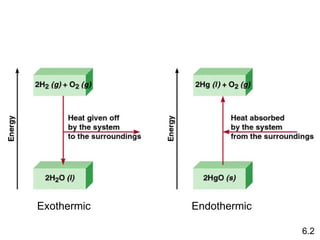



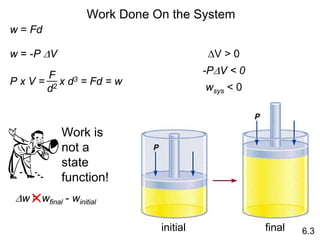
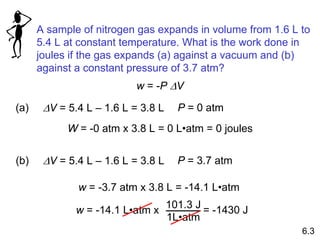

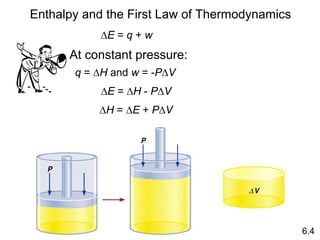
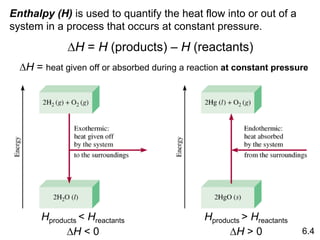
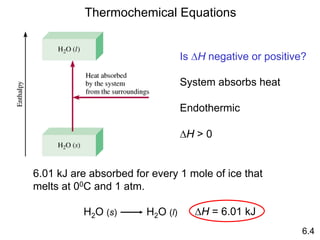
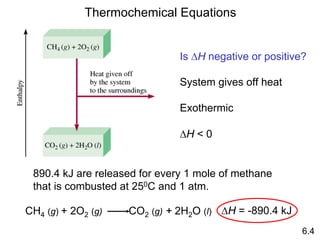
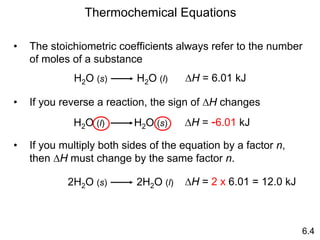
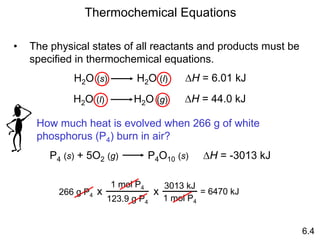

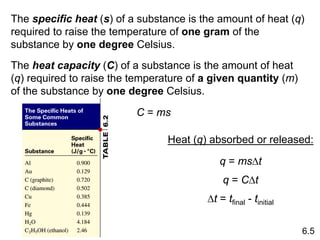

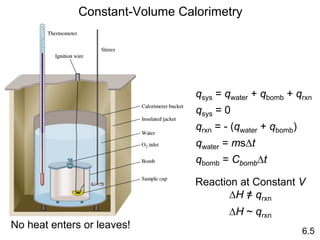
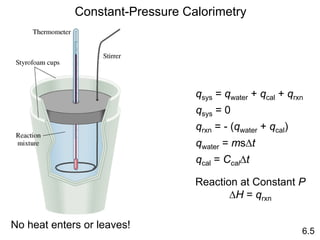
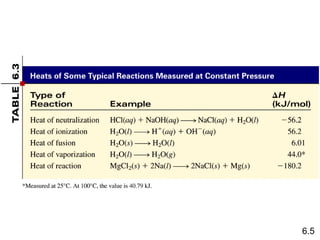
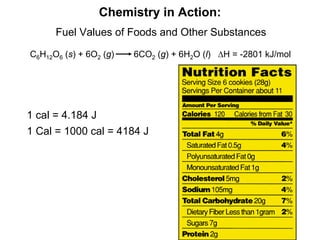
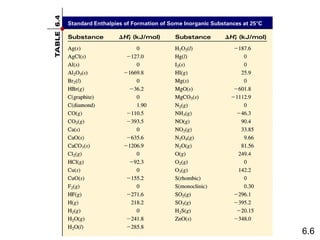
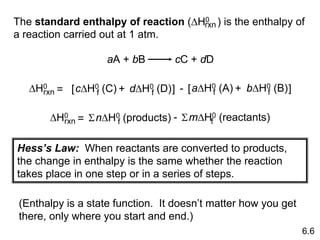
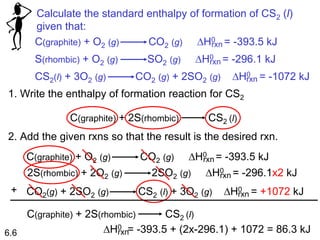
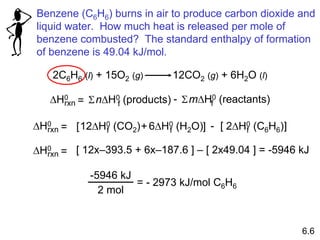
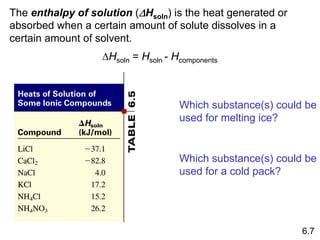
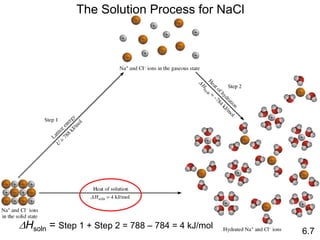

Thermochemistry is the study of heat changes in chemical reactions. There are several key concepts: 1) Exothermic reactions release heat to the surroundings and have a negative ∆H value, while endothermic reactions absorb heat from the surroundings and have a positive ∆H value. 2) The first law of thermodynamics states that energy is conserved and can be converted between different forms but not created or destroyed. ∆Esystem = q + w, where q is heat and w is work. 3) Enthalpy (H) is a state function that measures the heat absorbed or released during physical and chemical changes at constant pressure. The enthalpy change, ∆H































