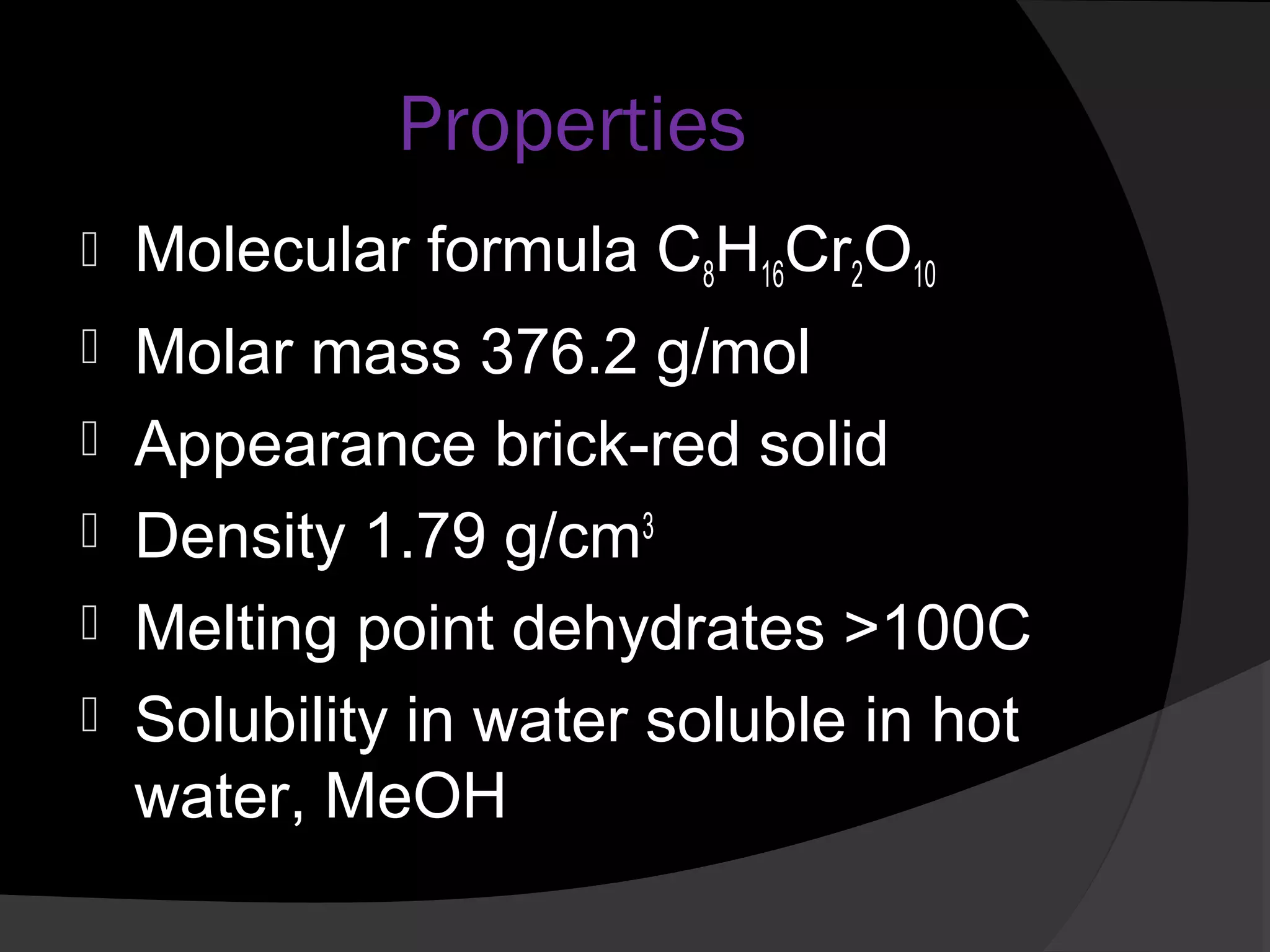
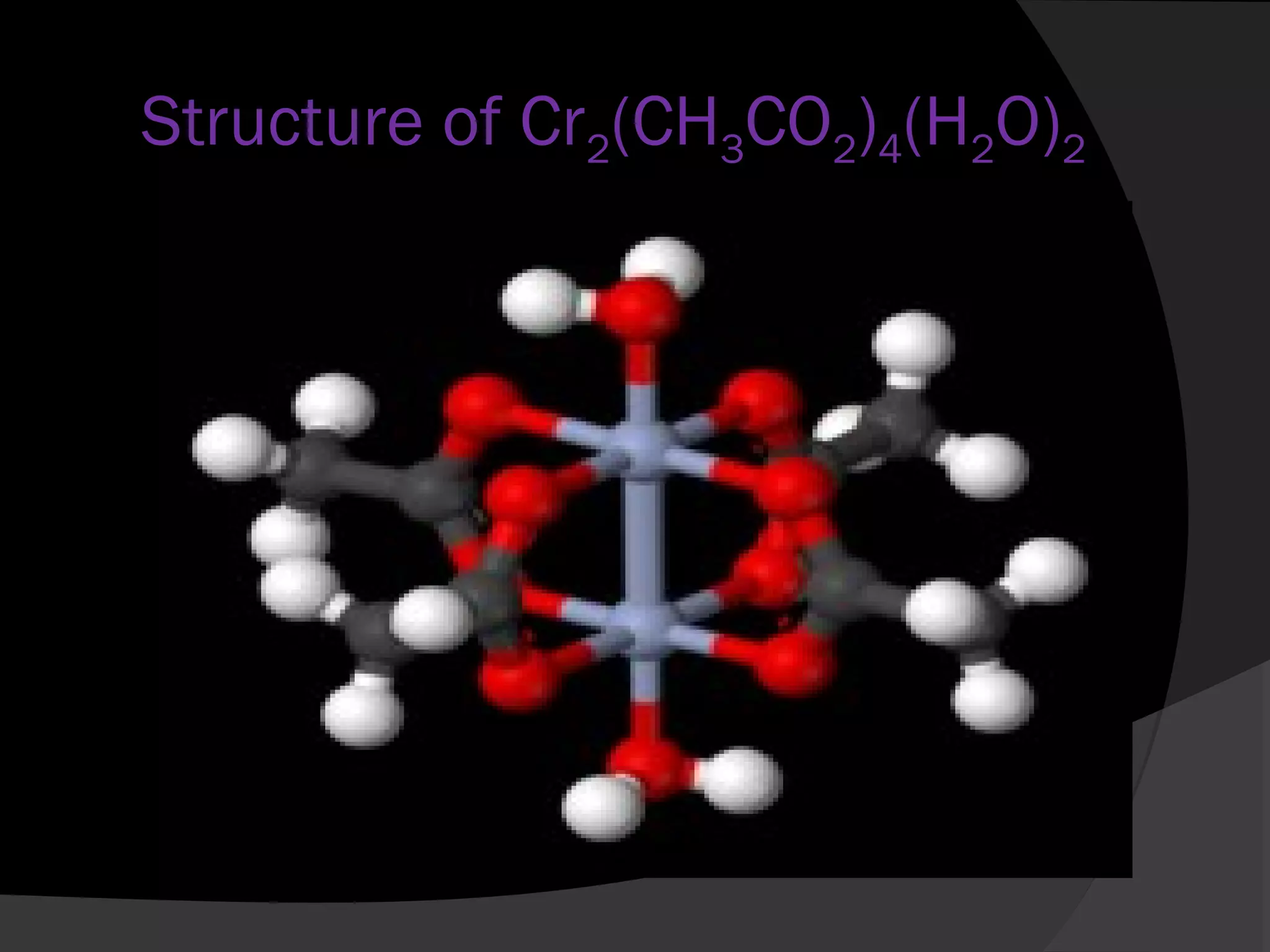
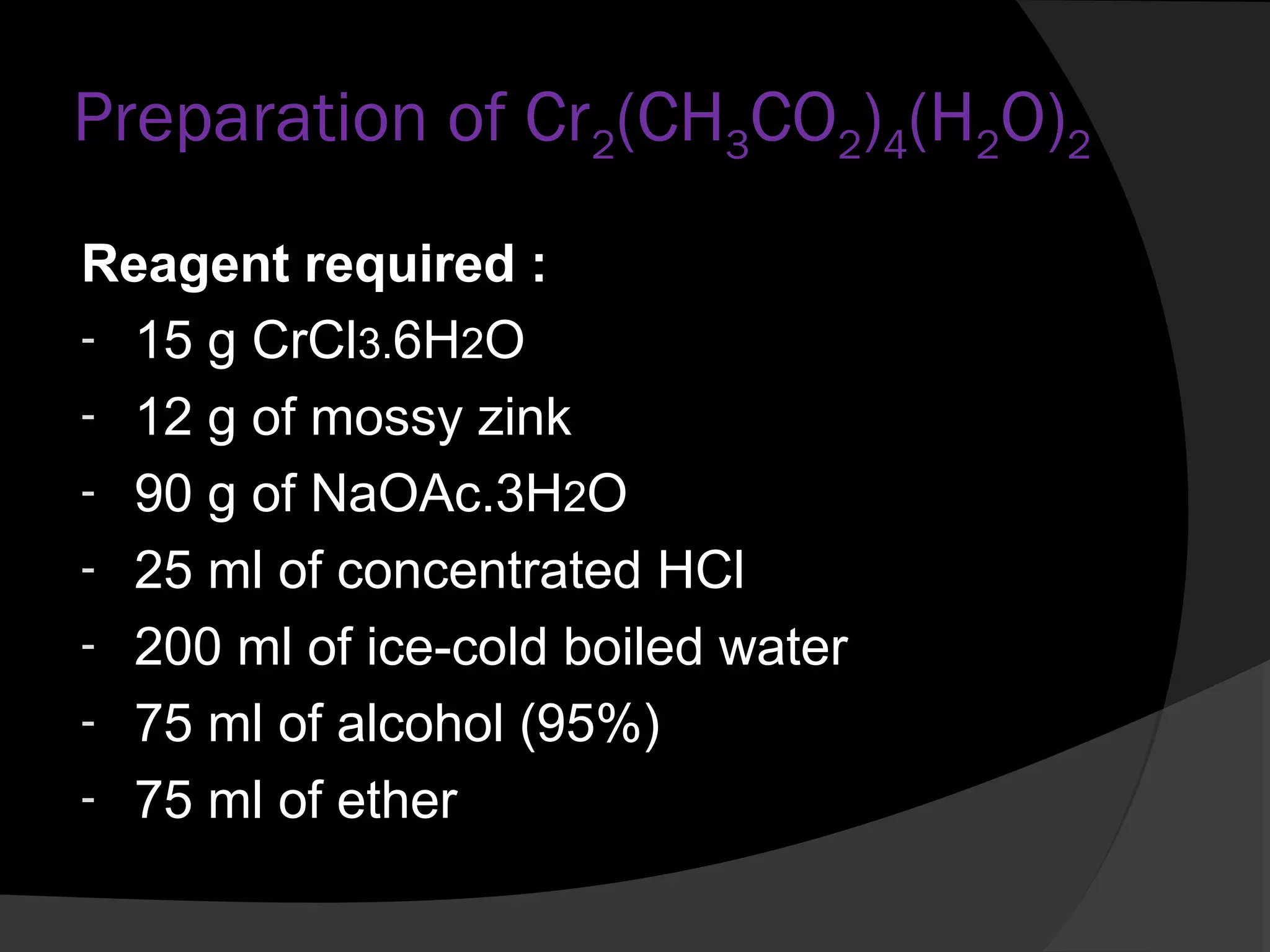
This document describes the properties and preparation of chromium(II) acetate hydrate. Chromium(II) acetate hydrate has the molecular formula C8H16Cr2O10 and appears as a brick-red solid. The document outlines the procedure to prepare chromium(II) acetate hydrate by reducing chromium(III) chloride with zinc in the presence of acetic acid and sodium acetate to form a red precipitate of the hydrate. Characterization details are provided, noting it will oxidize in air and is diamagnetic in its pure form.





![Characterization
If air is admitted to the sample, it will
gradually turn to the gray-green color
characteristic of the oxidized material.
Pure[Cr(OAc)2]2.2H2O is diamagnetic](https://image.slidesharecdn.com/synthesisofchromiumiiacetatehydrate-141124164558-conversion-gate01/75/Synthesis-of-chromium-ii-acetate-hydrate-9-2048.jpg)
![Analysis
[Cr(OAc)2]2·2H20 can be
analyzed by Spectroscopy
Infared (IR)](https://image.slidesharecdn.com/synthesisofchromiumiiacetatehydrate-141124164558-conversion-gate01/75/Synthesis-of-chromium-ii-acetate-hydrate-10-2048.jpg)
![Reaction
2 Cr3+ + Zn → 2 Cr2+ + Zn2+
2 Cr2+ + 4 OAc- + 2 H2O → Cr2(OAc)4(H2O)2
4 HO2CR + 2 Cr(C5H5)2 → Cr2(O2CR)4 + 4 C5H6
The overall net reaction is the sum of the above
reaction :
2CrCl3·6H2O + Zn + 4 NaOAc·3H2O
→[Cr(OAc)2]2·2H20 + ZnCl2 + 4 NaCl + 22 H20](https://image.slidesharecdn.com/synthesisofchromiumiiacetatehydrate-141124164558-conversion-gate01/75/Synthesis-of-chromium-ii-acetate-hydrate-11-2048.jpg)
